Published online Mar 14, 2005. doi: 10.3748/wjg.v11.i10.1540
Revised: September 30, 2004
Accepted: October 18, 2004
Published online: March 14, 2005
AIM: Racecadotril is a specific enkephalinase inhibitor that exhibits intestinal antisecretory activity without affecting intestinal transit. Loperamide is an effective anti-diarrheal agent, but it usually induces constipation. This study is to compare the efficacy, safety, and tolerability of racecadotril versus loperamide in the outpatient treatment of acute diarrhea in adults.
METHODS: A two-center, randomized, parallel-group, single-blind study was carried out to compare the efficacy, tolerability, and safety of racecadotril (100 mg thrics daily) and loperamide (2.0 mg 2 twice daily) in 62 adult patients suffering from acute diarrhea. The main efficacy criterion used was the duration of diarrhea after beginning the treatment (in hours). Other signs and symptoms were also evaluated.
RESULTS: The clinical success rates for these anti-diarrheal treatments were 95.7% and 92.0% for racecadotril and loperamide respectively. Patients on racecadotril had a median duration of diarrhea of 19.5 h compared with a median of 13 h for patients on loperamide. Rapid improvement in anal burn and nausea was found for each drug. However, more patients on loperamide suffered from reactive constipation (29.0% vs 12.9%). Itching, another adverse event was notably higher in the racecadotril group (28.6% vs 0%). With regard to other adverse events, the two medications showed similar occurrence rates and similar concomitant medication usage rates.
CONCLUSION: Racecadotril and loperamide are rapid, equally effective treatments for acute diarrhea in adults, but loperamide treatment is associated with a higher incidence of treatment-related constipation.
- Citation: Wang HH, Shieh MJ, Liao KF. A blind, randomized comparison of racecadotril and loperamide for stopping acute diarrhea in adults. World J Gastroenterol 2005; 11(10): 1540-1543
- URL: https://www.wjgnet.com/1007-9327/full/v11/i10/1540.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i10.1540
Infections of the gastrointestinal tract, especially infectious diarrhea, are among the most common debilitating infectious diseases, afflicting people of all ages around the world[1]. Diarrhea remains the third most frequent syndrome seen in general practice[2]. Although an etiologic agent is not found in many cases, microbial infection is the most common cause of most acute diarrheal diseases. The underlying pathophysiological problem of acute diarrhea is generally attributed to hypersecretion by the intestinal mucosa. Edelman commented that the ideal treatment for acute diarrhea should combine replacement of water and electrolytes with a medication able to inhibit intestinal hypersecretion while not slowing gastrointestinal transit[3]. Despite possessing effective anti-diarrheal properties, the μ-receptor agonist, loperamide and other opiates, may cause adverse effects such as reactive constipation and abdominal distension[4]. Their mode of action is through disrupting forward propulsive motility, increasing gut capacity, and delaying passage of fluid through the intestine.
The enkephalins were discovered in 1975, and act as neurotransmitters along the gastrointestinal tract where they are found in high levels in the mucosal cells[5]. Enkephalins, endogenous opiate substances contributing to antisecretory activity, play an important physiological role acting as neurotransmitters, notably along the digestive tract. These substances can elicit intestinal antisecretory activity without affecting intestinal transit. Racecadotril, a specific enkephalinase inhibitor, exhibits intestinal antisecretory activity not only in animal models but also in humans, without contributing to intestinal transit time[6]. Although several studies about this drug have been reported in the literature, no study has been reported in the oriental country; so this study was designed to compare the efficacy, safety and tolerability of racecadotril with loperamide in the treatment of acute diarrhea in adult patients.
Male or female adults over 18 years of age who were suffering from acute diarrhea of presumed infectious origin were eligible for inclusion in the study. Acute diarrhea was defined as the passing of at least 3 watery stools in a minimum of 24 h and for the duration of less than five days.
Exclusion criteria were the presence of chronic, iatrogenic, or bloody diarrhea, having received antibiotic treatment for other medical or surgical problems, having a history of renal or hepatic dysfunction, having a concomitant infection, or being otherwise immunocompromised. Patients receiving treatment with an anti-diarrheal drug in the five days prior to the study were also excluded. Pregnant or lactating women and women planning pregnancy were also ineligible for study participation.
The study was a randomized, single-blind, and parallel group design implemented in two separate centers in Taiwan from April 2001 to December 2001. Before treatment, the patients collected one stool for culture, and gave blood for a blood cell count. Patients were randomly allocated to receive either 100 mg tablets of racecadotril three times daily (half an hour before or one hour after meal), or 2.0 mg tablets of loperamide twice daily.
The drugs are prepared with the same color of capsule in the outer appearance and given (drugs) by pharmacist according to a randomized controlled (sheet) schedule.
Patients were treated until recovery, defined as the production of 2 consecutive normal stools or no stool production for a period of 12 h. If recovery did not occur in 7 d, this treatment was discontinued. The first dose of the medication was taken under the supervision of a designated study physician or nurse.
No additional anti-diarrheal therapies or concomitant medications were permitted during the study.
Efficacy was documented by the physician and in a diary card filled in by the patient. The time, number, and stool characteristics were recorded, as were the occurrence of several adverse signs and symptoms. Formal evaluation by the physician occurred on conclusion and at the end of the treatment visit occurring 10-14 d after entering the study. Patients withdrawn from the study were to attend a follow-up visit three weeks after withdrawal to monitor adverse experiences and concomitant medication changes.
The primary efficacy criterion was the duration of diarrhea in hours, from the first treatment dose to recovery. Secondary efficacy criterion consisted of duration of abdominal pain and abdominal distension. The overall clinical response as a success or failure was assessed by physicians.
Tolerability and safety were assessed by recording the adverse events experienced during treatment and by the occurrence of constipation. The signs and symptoms evaluated were pain on abdominal palpation, anorexia, nausea, and anal burning.
The study was approved by the Institutional Review Board or Ethics Committee prior to each center’s initiation and conducted in accordance with ICH and the local Government’s Clinical Practices and the Declaration of Helsinki; all patients gave their informed consent.
Statistical analysis was performed using SAS vision 8. Both intent-to-treat (ITT) and per protocol (PP) analyses were performed for primary and secondary parameters. Estimates of the survival distribution of the duration of diarrhea were analyzed using Kaplan-Meier survival analysis techniques. The overall clinical response of treatment groups was compared using the Cochran-Mantel-Haenszel test, as was the comparison of treatment group changes from inclusion to the end of treatment visit.
A total of 62 patients were entered into the study, and all received at least one dose of study medication under supervision at the investigator site. All patients who had valid baseline data and were used in the ITT analysis are shown in Table 1.
| Characteristics | Racecadotril (n = 31) | Loperamide (n = 31) | P |
| Male/Female | 15 (48.4)/16 (51.6%) | 17 (54.8%)/14 (45.2) | 0.7997 |
| Oriental | 31 (100%) | 31 (100%) | 1 |
| Age (yr) | 38.4±15.1 | 34.7±12.3 | 0.2961 |
| Weight (kg) | 57.4±12.7 | 59.6±9.0 | 0.4339 |
| Height (cm) | 162.6±9.6 | 164.8±9.6 | 0.357 |
| BMI | 21.5±3.3 | 22.0±2.9 | 0.6074 |
The full data set consisted of 62 patients, with the 48 patients who participated fully making up the per protocol population (77.4%). Two patients (6.5%) did not complete the study due to adverse experiences, 11 patients (17.7%) were considered non-completers due to deviation from protocol, and 2 patients (3.2%) were lost to follow-up.
Of the initial 31 patients who received racecadotril, there were 15 males and 16 females. Thirty-one received loperamide (17 males and 14 females). The mean ages were 38.4±15.1 years for racecadotril and 34.7±12.3 years for loperamide. Patients in the 2 groups were comparable on other demographic variables (Table 1).
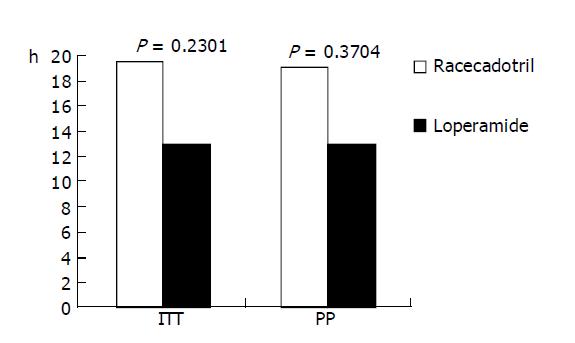
Based on the ITT population, the mean duration of diarrhea in the racecadotril group (n = 31) was 19.5 h and for the loperamide group (n = 31) was 13.0 h (P = 0.23). Based on PP analysis, the mean duration of diarrhea was 19 h in the racecadotril group (n = 23) compared to 13.0 h for the loperamide group (n = 25, P = 0.37). Both results are shown in Figure 1.
The mean duration of abdominal pain in the ITT population was 16 h for racecadotril and 14 h for loperamide. In the PP population, the median duration of abdominal pain was 16 h for racecadotril and 15 h for loperamide. The median duration of abdominal distension for the racecadotril group was 12 h in both the ITT and the PP analyses, but 12 and 14 h respectively in these populations on loperamide (Table 2).
| Symptom | Population | Racecadotril | Loperamide | P |
| Abdominal pain | ITT | 16 | 14 | 0.9509 |
| PP | 16 | 15 | 0.7177 | |
| Abdominal distension | ITT | 12 | 14 | 0.5602 |
| PP | 12 | 12 | 0.525 |
Therapeutic improvement rates in the anal burning sensation were 71.0% (ITT) and 78.3% (PP) in the racecadotril group; 74.2% (ITT) and 76.0% (PP) in the loperamide group. For symptoms of nausea, the clinical improvement rates were 74.2% (ITT) and 78.3% (PP) in racecadotril group, 77.4% (ITT) and 80.0% (PP) in loperamide group (Table 3).
| Symptom | Population | Racecadotril (%) | Loperamide (%) | P |
| Anal burn | ITT | 22/31 (71.0) | 23/31 (74.2) | 0.7353 |
| PP | 18/23 (78.3) | 19/25 (76.0) | 0.7428 | |
| Nausea | ITT | 23/31 (74.2) | 24/31 (77.4) | 0.659 |
| PP | 18/23 (78.3) | 20/25 (80.0) | 0.6475 |
Treatment in the majority of patients was judged clinically successful. The percentages of successful treatments were 87.1% (ITT) and 95.6% (PP) in the racecadotril group and 87.1% (ITT) and 92.0% (PP) in the loperamide group (Table 4).
| Population | Racecadotril (%) | Loperamide (%) | P |
| ITT | |||
| Clinical success | 22/31 (87.1) | 27/31 (87.1) | 0.7212 |
| PP | |||
| Clinical success | 22/23 (95.6) | 23/25 (92.0) | 0.6248 |
There were 14 (24.0%) patients who experienced at least one adverse event during the study: 8 (25.0%) in the racecadotril group and 7 (22.0%) in the loperamide group. The most frequently occurring adverse events were constipation (16.7%), bloody stool (11.1%), and itching (11.1%). A significantly greater number of patients experienced constipation in the loperamide treatment group (29.0% vs 12.9%, Table 5).
| Population event | Racecadotril (n = 31) (%) | Loperamide (n = 21) (%) |
| Constipation | 4 (12.9) | 9 (29.0) |
| Bloody stool | 1 (14.2) | 1 (9.1) |
| Skin itching | 2 (28.6) | 0 (0.0) |
| Abdominal pain on palpation | 1 (14.3) | 0 (0.0) |
The results of this randomized, parallel, controlled study confirm that the efficacy, tolerability, and safety of racecadotril are comparable to those of loperamide in treating acute diarrhea in adults, but racecadotril treatment is less associated with the adverse event of constipation.
In clinical practice, we usually use anti-diarrheal agents for patients suffering from acute diarrhea. In principle, because the mechanisms of stopping diarrhea are different in loperamide and racecadotril, there might be reason to choose one before the other. Loperamide activates the μ-receptor, prolonging the orocecal and colonic transit times by disrupting the gut’s electrical activity, increasing gut capacity, and delaying the passage of fluids through the small intestine; it has no direct effect on absorption[12]. Racecadotril is a specific inhibitor of enkephalinase. It activates the δ-receptor to reduce secretory activity in the gut[16], thereby prolonging the antisecretory effect of the endogenous enkephalins.
Of the 62 patients randomized (ITT population), 48 patients (23 in racecadotril group and 25 in loperamide group) were considered valid as per protocol. The results of the study showed that no statistically significant differences were found in the effects of these medications on the duration of diarrhea (19.5 h vs 13.0 h), the duration of abdominal pain (P = 0.95, ITT and P = 0.71, PP), or on the duration of abdominal distension (P = 0.56, ITT and P = 0.52, PP) for the racecadotril groups and the loperamide groups respectively. The clinical improvement rates in anal burning sensation and nausea were greater than seventy percent in both the racecadotril group and the loperamide group, but the differences between the two groups did not reach statistical significance.
Therefore, the estimated clinical success rates, including duration of abdominal pain, abdominal distension, diarrhea, and anal burning sensation, were high in both the racecadotril and loperamide treatment groups in per protocol populations (95.6% and 92.0% respectively).
These two different medications show similar adverse events such as constipation, bloody stool, abdominal pain, skin itching, palpitation, dizziness, cold sweating, and headache. Skin itching was somewhat more frequent in the racecadotril group, but there was no statistically significant difference. This may be due to the relatively small study population, and needs further confirmation with a larger population. The adverse event of constipation in the racecadotril group is lower than in the loperamide treated group (12.9% and 29.0%) although there was no statistical significance.
The in vivo study by Hinterleitner et al also showed that plasma enkephalinase was significantly inhibited within the first 30 min of administration of racecadotril, and maximum inhibition was seen after 60 min. The inhibition of intestinal fluid secretion by racecadotril was confirmed by studying the effect of racecadotril on cholera-induced hypersecretion in the jejunum of 6 healthy subjects, which showed that racecadotril had no influence on basal water and electrolyte absorption (133 vs 140 mL/30 cm × h). But in control group, significant water secretion was induced (131 mL/30 cm × h). Racecadotril completely prevented this secretion by leaving an absorption rate of 27 mL/30 cm × h[17].
There also demonstrated inhibition of intestinal secretion by racecadotril in diarrhea induced by castor oil, a model of hypersecretory diarrhea[7]. In a study by Hamza et al[18], racecadotril produced a significant (P = 0.025) decrease in stool weight during the first day of treatment compared with placebo, and was also associated with significantly fewer diarrhoeic stools than placebo after 1 d of treatment (P = 0.027), but less abdominal distension was found on racecadotril group than placebo group (5.6% vs 18.2%).
The anti-motility mechanism of action of many traditional drugs used to treat diarrhea can lead to adverse effects such as constipation, abdominal pain, and abdominal distension, which limits the potential use of these drugs[14,19,20]. This study revealed that racecadotril is associated with a somewhat lower incidence of treatment-related constipation than that of loperamide. The study of the result in Rouge’ et al showed racecadotril and loperamide were both rapidly and similarly effective, diarrhea resolving in both cases in nearly 2 d. With racecadotril, however, abdominal distension vanished significantly more rapidly (50.0% vs 27.0%; P<0.05), and reactive constipation was less frequent (31.1% vs 8.1%; P<0.02). These differences can be accounted for by the distinct mechanisms of antidiarrheal activity of the two drugs[8]. In our study the mean duration of stopping diarrhea is 19.5 h on racecadotril treated group that is better than Rouge’ et al study. So this study showed racecadotril has better effective for stopping diarrhea in oriental population, but has the same safety between oriental population and western population. However, multicenter-trial with a larger cohort of patients are required before racecadotril can be recommended as the drug of choice in acute diarrhea in oriental population.
In summary, the results of our study have confirmed that racecadotril is an effective antihypersecretory agent for the safe, outpatient treatment of acute diarrhea in adults, and are consistent with a previous study in showing a lower incidence of treatment-related constipation for this medication, compared to loperamide.
Edited by Guo SY Language Editor Elsevier HK
| 1. | Sheeby TW. Digestive disease as a national problem. VI. Enteric disease among United States troops in Vietnam. Gastroenterology. 1968;55:105-112. [PubMed] |
| 2. | Sprinz H. Pathogenesis of intestinal infections. Arch Pathol. 1969;87:556-562. [PubMed] |
| 3. | Edelman R. Prevention and treatment of infectious diarrhea. Speculations on the next 10 years. Am J Med. 1985;78:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Schiller LR, Santa Ana CA, Morawski SG, Fordtran JS. Mechanism of the antidiarrheal effect of loperamide. Gastroenterology. 1984;86:1475-1480. [PubMed] |
| 5. | Pollard H, Moreau J, Ronco P, Verroust P, Schwartz JC. Immunoautoradiographic localisation of enkephalinase (EC 3.4.24.11) in rat gastrointestinal tract. Neuropeptides. 1991;19:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Primi MP, Bueno L, Baumer P, Berard H, Lecomte JM. Racecadotril demonstrates intestinal antisecretory activity in vivo. Aliment Pharmacol Ther. 1999;13 Suppl 6:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Baumer P, Danquechin Dorval E, Bertrand J, Vetel JM, Schwartz JC, Lecomte JM. Effects of acetorphan, an enkephalinase inhibitor, on experimental and acute diarrhoea. Gut. 1992;33:753-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Roge J, Baumer P, Berard H, Schwartz JC, Lecomte JM. The enkephalinase inhibitor, acetorphan, in acute diarrhoea. A double-blind, controlled clinical trial versus loperamide. Scand J Gastroenterol. 1993;28:352-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 9. | Vetel JM, Berard H, Fretault N, Lecomte JM. Comparison of racecadotril and loperamide in adults with acute diarrhoea. Aliment Pharmacol Ther. 1999;13 Suppl 6:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Lecomte JM. An overview of clinical studies with racecadotril in adults. Int J Antimicrob Agents. 2000;14:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Prado D. A multinational comparison of racecadotril and loperamide in the treatment of acute watery diarrhoea in adults. Scand J Gastroenterol. 2002;37:656-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Basilisco G, Camboni G, Bozzani A, Paravicini M, Bianchi PA. Oral naloxone antagonizes loperamide-induced delay of orocecal transit. Dig Dis Sci. 1987;32:829-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Kachel G, Ruppin H, Hagel J, Barina W, Meinhardt M, Domschke W. Human intestinal motor activity and transport: effects of a synthetic opiate. Gastroenterology. 1986;90:85-93. [PubMed] |
| 14. | Ruppin H. Review: loperamide--a potent antidiarrhoeal drug with actions along the alimentary tract. Aliment Pharmacol Ther. 1987;1:179-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Schiller LR, Santa Ana CA, Morawski SG, Fordtran JS. Mechanism of the antidiarrheal effect of loperamide. Gastroenterology. 1984;86:1475-1480. |
| 16. | Shook JE, Lemcke PK, Gehrig CA, Hruby VJ, Burks TF. Antidiarrheal properties of supraspinal mu and delta and peripheral mu, delta and kappa opioid receptors: inhibition of diarrhea without constipation. J Pharmacol Exp Ther. 1989;249:83-90. [PubMed] |
| 17. | Hinterleitner TA, Petritsch W, Dimsity G, Berard H, Lecomte JM, Krejs GJ. Acetorphan prevents cholera-toxin-induced water and electrolyte secretion in the human jejunum. Eur J Gastroenterol Hepatol. 1997;9:887-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Vetel JM, Berard H, Fretault N, Lecomte JM. Comparison of racecadotril and loperamide in adults with acute diarrhoea. Aliment Pharmacol Ther. 1999;13 Suppl 6:21-26. |