Published online Mar 14, 2005. doi: 10.3748/wjg.v11.i10.1515
Revised: September 24, 2004
Accepted: November 19, 2004
Published online: March 14, 2005
AIM: To investigate the effects of anti-fibrosis I herbal compound on intracellular Ca2+ in activated hepatic stellate cell (HSC) and to try to survey its molecular mechanism in treatment and prevention of hepatic fibrosis and portal hypertension.
METHODS: The activated HSC line was plated on small glass cover slips in 24 wells culture dishes at a density of 5×106 /mL, and incubated in RPMI-1640 media for 24 h. After the cells were loaded with Fluo-3/AM, intracellular Ca2+ was measured with laser scanning confocal microscopy (LSCM). The dynamic changes of intracellular Ca2+, stimulated by carbon tetrachloride, TGF-β1 antibody and the drug serum of anti-fibrosis I herbal compound and under orthogonal design were determined by LSCM. The effect of anti-fibrosis I herbal compound on intracellular Ca2+ was observed before and after the addition of TGF-β1 antibody.
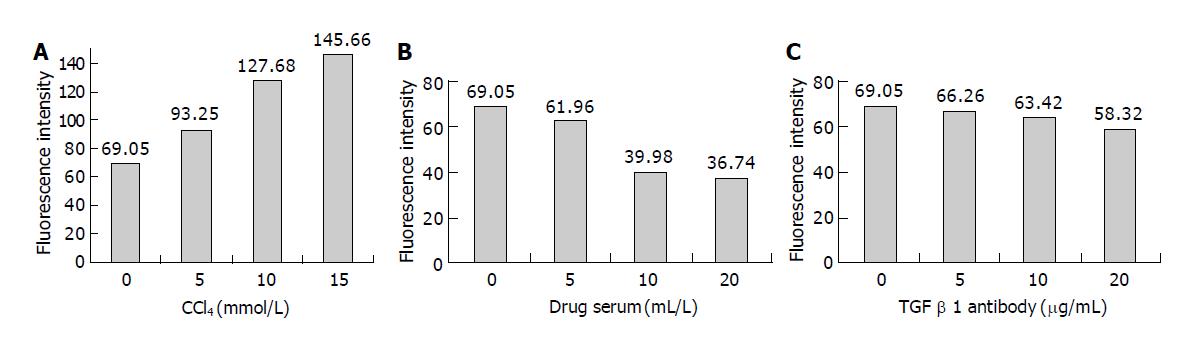
RESULTS: The intracellular Ca2+ were significantly different in different dosage of carbon tetrachloride anti-fibrosis I formula drug serum, TGF-β1 antibody and different turn of these substance, but their interval time between CCl4 and TGF-β1 antibody, CCl4 and anti-fibrosis I drug serum had no influence on intracellular Ca2+. The result showed intracellular Ca2+ wasn’t significantly different between rat serum without anti-fibrosis I and untreated group. After carbon tetrachloride stimulation, intracellular Ca2+ of activated HSC increased significantly when the dosage of CCl4 from 5 to 15 mmol/L, however, decreased significantly after stimulation by 5-20 μg/mL TGF-β1 antibody or 5-20 mL/L drug serum. Moreover, before and after the addition of TGF-β1 antibody, intracellular Ca2+ was significantly different. These results suggested that the molecular mechanism was independent of blocking TGF-β1 effects.
CONCLUSION: Anti-fibrosis I herbal compound may treat hepatic fibrosis and decrease portal hypertension by inhibiting activated HSC contractility through decrease of intracellular Ca2+.
- Citation: Xiao YH, Liu DW, Li Q. Effects of drug serum of anti-fibrosis I herbal compound on calcium in hepatic stellate cell and its molecular mechanism. World J Gastroenterol 2005; 11(10): 1515-1520
- URL: https://www.wjgnet.com/1007-9327/full/v11/i10/1515.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i10.1515
Hepatic fibrosis, which may ultimately lead to cirrhosis, is the pathological base of all the chronic hepatic diseases and is characterized by the net accumulation of extracellular matrix (ECM), including collagen, glycoproteins, and proteoglycans[1]. Lots of data have indicated that the activation of hepatic stellate cell (HSC) is the cytological base of the hepatic fibrosis. Activated HSC plays an important role in the regulation of intrahepatic resistance and microhemodynamics, because of its smooth muscle characteristics, in particular, its contractility[2,3]. Contractility of HSCs is regulated by intracellular Ca2+ and certain vasoactive factors[3]. Hence, activated HSCs have become a therapeutic target for chronic liver disease. The activation of HSCs is the effect of various cell factors by side secretion or self-secretion, of which, transforming growth factor-β1 (TGF-β1) is a key molecule responsible for tissue fibrosis and provides a basis for targeting TGF-β1 as an anti-fibrotic agent[4-6]. In the past years, marked progress in the understanding of the pathophysiology of hepatic fibrosis and portal hypertension has opened the door to pharmacological treatments, resulting in dramatic changes in the therapeutic approaches to hepatic fibrosis and portal hypertension. However, few effective drugs can slow the progression of the fibrosis. In the recent study, we have proved from animal experiment that anti-fibrosis I compound have the function of anti-hepatic fibrosis induced by carbon tetrachloride (CCl4) in rats. This study was performed, using the serum pharmacological method, giving drugs to animals, separating serum with drug and putting them in cultured cells in vitro, then the change in intracellular calcium ([Ca2+]i) was observed with laser scanning confocal microscope for further studying mechanism of Chinese medicine.
Anti-fibrosis I herbal compound included Salvia miltiorrhiza, Sparganium stoloniferum, Angelica sinensis, Amyda sinensis, Curcuma aromatica, Carex phacota. These were purchased from Shijiazhuang Lerentang Pharmacy, China. The herbs were boiled with water and extracted by alcohol: put 95% alcohol in liquid of anti-fibrosis herbs, mixed the alcohol and herbs, filtrate the protein and amylum, then heated the liquid at 90-95 °C to evaporate the remaining alcohol, took the solution of crude drug at 2.5 g/mL final density, which were infused from esophagus into stomach of Wistar rats from Animal Center of Hebei Medical University (Shijiazhuang, China), weighing about 300-400 g, once a day for 3 d, the dose was about 7 times to adult dose whose body weight was 65 kg. After infusion of the drug for 2 h in the last time, rats were given the drug again, 1 h later, blood was collected from inferior vena cava, centrifuged at 2000 r/min for 15 min to get serum, which was put at 56 °C for 30 min to get rid of other possible biological living substance, then removed bacteria by 0.22 μm millipore filtration membrane, sealed up and stored in the -20 °C for further use. Meanwhile, rat serum without anti-fibrosis I was prepared for the negative control.
Polyclonal rabbit antibody (Ab) to rat TGF-β1 was bought from Santa Cruz Biotechnology, INC; RPMI-1640 culture medium was purchased from American Gibco Company; Fetal bovine serum was purchased from Sijiqing Biological Products Company (Hangzhou, China); Carbon tetrachloride (CCl4) was purchased from Tianjin Reagent Factory; Fluo-3/AM(1-[2-Amino-5-(2,7-dichloro-6-hydroxy-3-oxo-9-xanthenyl) phenoxy]-2-(2-amino-5-methylphenoxy) ethane-N, N,N’,N’-tetraacetic acid/acetoxymethyl ester), pluronic F-127 were purchased from American Biotium Company; 0.25 % trypsin digestive reagent, dimethylsulfoxide (DMSO), 2-[4-(2-Hydroxyethyl)-1-piperazinyl] ethanesulfonic acid (HEPES) were bought from American Sigma Company.
Main instrument: Laser scanning confocal microscopy (LSCM) (Leica DM IRBE); Bechtop (Suzhou Purify Equipment Factory), HH CP-T Type CO2 incubators (Shanghai Fuma Experiment Equipment Company Limited); Inverted Microscope (Chongqin optical instrument factory).
Buffer solution (mmol/L): NaCl 130, KCl 2.7, CaCl2 1.5, MgCl2 2, HEPES 10, glucose 10, pH 7.4 (NaOH).
HSC strain-CFSC was created and donated by professor Greenwel, whose phenotype was activated HSCs. It was separated from rats with hepatocirrhosis induced by CCl4, and was well-cultured to gain eternal life[7]. HSCs frozen in liquid nitrogen were rapidly revivified in a 37 °C water bath, then incubated in the RPMI-1640 media containing 10 mL/L fetal bovine serum, 100 U/mL pencillin, 100 U/mL streptomycin sulfate, and cultured at 37 °C, 5 mL/L carbon dioxide (CO2) condition. Cells were digested by 2.5 g/L trypsin to subculture. Suspending Liquid of cells at a density of 5×106 cells/mL was plated on small glass cover slips in 24 wells culture plate, cultured in a 37 °C, 5 mL/L CO2 incubator. These cultured cells were used in the following experiments.
HSCs were handled with CCl4, rat serum, drug serum, TGF-β1 antibody for 24 h, respectively, whose dosage CCl4 was 0, 5, 10, 15 mmol/L, both rat serum and drug serum (volume fraction) were 0, 5, 10, 20 mL/L , TGF-β1 antibody was 0, 5, 10, 20 μg/mL, 0 was the control group, and loaded with Fluo-3/AM.Then orthogonal trial of mixed level was designed using spss/11.5 software, factors-levels were shown in Table 1, the plans of trial in Table 2. After adding second factor for 24 h, HSCs were loaded with Fluo-3/AM. Using the method of range analysis for orthogonal trial, the best level of every factor was chosen. Firstly HSCs was treated with CCl4 for certain time, before and after addition of TGF-β1 antibody for certain time, rat serum or drug serum was added into the cells, 24 h later and HSCs were loaded with Fluo-3/AM.
| Factor | |||||
| Level | CCl4(mmol/L) | Durg serum(mL/L) | TGF-β1 Ab(mg/mL) | Time1(h) | Turn |
| 1 | 5 | 5 | 5 | 0.5 | Adding CCl4 firstly |
| 2 | 10 | 10 | 10 | 2 | Adding durg serum/TGF-β1 Ab firstly |
| 3 | 15 | 20 | 20 | 4 | |
| Trial | CCl4(mmol/L) | Drug serum(mL/L) | TGF-β1 Ab(mg/mL) | Time(h) | Turn | Fluorescence intensity | |
| TGF-β1 Ab | Drug serum | ||||||
| 1 | 5 | 5 | 5 | 0.5 | 1 | 81.03±7.36 | 78.25±6.84 |
| 2 | 15 | 20 | 20 | 0.5 | 1 | 126.52±13.54 | 109.38±15.63 |
| 3 | 5 | 10 | 10 | 2 | 1 | 75.84±6.81 | 72.54±9.52 |
| 4 | 10 | 20 | 20 | 2.0 | 1 | 106.46±10.23 | 88.65±10.38 |
| 5 | 10 | 5 | 5 | 4 | 1 | 117.65±9.26 | 102.13±12.45 |
| 6 | 15 | 10 | 10 | 4 | 1 | 138.24±12.57 | 113.36±11.82 |
| 7 | 10 | 10 | 10 | 0.5 | 2 | 94.53±8.67 | 84.47±8.23 |
| 8 | 15 | 10 | 10 | 0.5 | 2 | 128.67±11.56 | 96.48±9.65 |
| 9 | 15 | 5 | 5 | 2 | 2 | 138.21±14.83 | 118.65±12.34 |
| 10 | 5 | 20 | 20 | 2 | 2 | 62.17±6.58 | 50.16±6.48 |
| 11 | 10 | 5 | 5 | 4.0 | 2 | 114.28±8.37 | 92.54±8.75 |
| 12 | 5 | 20 | 20 | 4 | 2 | 64.23±5.26 | 46.23±3.64 |
The cells were loaded with 10 μmol/L Fluo-3/AM for 30 min at 37 °C under a mixed gas containing 95 mL/L air and 5 mL/L CO2, washed 2-3 times with buffer solution, then put in 1 mL buffer solution micro-incubation perfusion chamber. One or two cell and their layers were chosen to observe with LSCM. Excitation wave of Fluo-3 was 488 nm and emission wave 525 nm. Fluorescence intensity of [Ca2+]i in eight cells chosen randomly was observed using TCS SP2 with LSCM to calculate the average fluorescence intensity of the whole cell. The dynamic changes of [Ca2+]i in individual HSC after stimulation with either CCl4, anti-fibrosis I drug serum, or TGF-β1 antibody were recorded using LSCM and a computer. The concentration of HSC [Ca2+]i was measured in terms of intracellular fluorescent intensity. This study recorded the relative value of fluorescence intensity for the change of [Ca2+]i. The larger the fluorescence intensity, the higher the [Ca2+]i concentration.
All data were expressed as mean±SD and analyzed with spss/11.5 statistic software. The mean difference was calculated using the analysis of variance (ANOVA) for orthogonal design, one-way ANOVA and Student-Newman-Keuls test for multiple comparison. The level of significance of hypothesis testing was P<0.05.
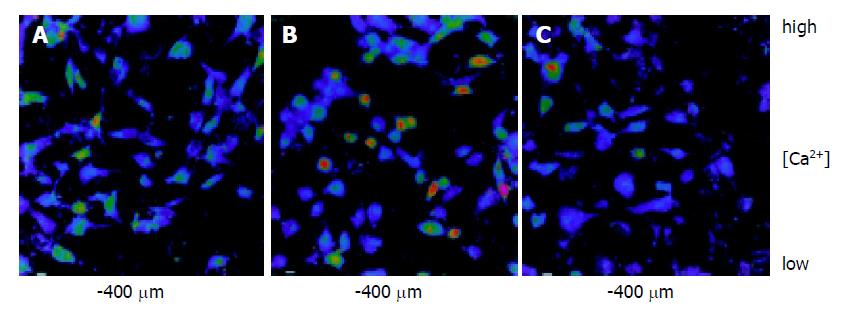
In the 5 min during observation, there is no obvious change of fluorescence intensity of HSCs, which in untreated group was 69.05±3.16. In rat serum group, [Ca2+]i was not significantly different when the dosage of rat serum was 0, 5, 10, 20 mL/L. While, CCl4 sharply increased the [Ca2+]i when the dosage of CCl4 from 5 to 15 mmol/L. CCl4 could make [Ca2+]i of HSCs overload, fluorescence intensity increase, what is more, the higher the CCl4 dosage, the stronger the fluorescence intensity, the higher the [Ca2+]i concentration (F = 760.602, P<0.05) (Figure 1A); After treating HSCs with 5-20 mL/L drug serum, the [Ca2+]i dropped (F = 554.962 , P<0.05); with 5-20 μg/mL TGF-β1 antibody, the [Ca2+]i did too (F = 39.393 , P<0.05). The higher drug serum or TGF-β1 antibody dosage, the less [Ca2+]i concentration (Figure 1B, Figure 1C). The change of fluorescence intensity of HSCs was shown (Figure 2A-Figure 2C). Compared with the untreated group, fluorescence intensity of HSCs treated with 10 mmol/L CCl4 was obviously strong, of which HSCs treated with drug serum of anti-fibrosis I herbal compound was weak.
The results were shown in Tables 2, 3. From Tables 2, 3, the concentration of [Ca2+]i were significantly different in different dosage of CCl4, anti-fibrosis I drug serum, TGF-β1 antibody and different turn of these substances, but there is no significance in their interval time between CCl4 and TGF-β1 antibody, CCl4 and anti-fibrosis I drug serum. The results of orthogonal design showed that the most important matter that affected [Ca2+]i was CCl4, the second important were anti-fibrosis I drug serum and TGF-β1 antibody (Table 4).
| Sources of variation | Sum of squares of deviation from mean | Degree of freedom | Variance | F | P |
| Variance analysis of TGF-β1 Ab | |||||
| CCl4 | 6933.936 | 2 | 3466.968 | 392.639 | 0 |
| TGF-b1 Ab | 378.054 | 2 | 189.027 | 21.408 | 0.007 |
| Time | 67.786 | 2 | 33.893 | 3.838 | 0.117 |
| Turn | 158.777 | 1 | 158.777 | 17.982 | 0.013 |
| Error | 35.320 | 4 8. | 830 | ||
| Variance analysis of drug serum | |||||
| CCl4 | 3969.848 | 2 | 1984.924 | 139.437 | 0 |
| Drug serum | 656.001 | 2 | 328.000 | 23.041 | 0.006 |
| Time | 55.274 | 2 | 27.637 | 1.941 | 0.257 |
| Turn | 478.551 | 1 | 478.551 | 33.617 | 0.004 |
| Error | 56.941 | 4 | 14.235 | ||
| Level | Drug serum | TGF-β1 Ab | ||||||
| CCl4 | Drug serum | Time | Turn | CCl4 | TGF-β1 Ab | Time | Turn | |
| 1 | 61.80 | 97.89 | 92.15 | 94.05 | 70.82 | 112.79 | 107.69 | 107.62 |
| 2 | 91.95 | 91.71 | 82.50 | 81.42 | 108.23 | 109.32 | 95.67 | 100.35 |
| 3 | 109.47 | 73.61 | 88.57 | 132.91 | 89.85 | 108.6 | ||
| R | 47.67 | 24.28 | 9.65 | 12.63 | 37.41 | 19.47 | 12.93 | 7.27 |
According to results of Orthogonal trial, the method of range analysis was used to find the best level of each factor, which was 15 mmol/L CCl4, 20 mL/L drug serum, 20 μg/mL TGF-β1 antibody. Firstly HSCs were treated with 15 mmol/L CCl4 for 0.5 h, before and after addition of TGF-β1 antibody for 2 h, drug serum was added into HSCs ,24 h later, the change of [Ca2+]i was observed. The results were shown in Table 5. Group 1: HSCs were treated with 15 mmol/L CCl4 for 0.5 h, added 20 mL/L drug serum; Group 2: HSCs were treated with 15 mmol/L CCl4 for 0.5 h, added 20 μg/mL TGF-β1 antibody; Group 3: HSCs were treated with 15 mmol/L CCl4 for 0.5 h, after addition of 20 mL/L drug serum for 2 h, add 20 μg/mL TGF-β1 antibody; Group 4: HSCs were treated with 15 mmol/L CCl4 for 0.5 h, after addition of 20 μg/mL TGF-β1 antibody for 2 h, add 20 mL/L drug serum. The results of one-way ANOVA showed [Ca2+]i in HSC treated with drug serum, before and after the addition of TGF-β1 antibody, were significantly different and also significantly different for multiple comparison.
| Group Methods of treatment | Fluorescence intensity | |
| 1 | CCl4+HSC+drug serum | 109.38±15.63 |
| 2 | CCl4+HSC+TGF-β1 Ab | 126.52±13.54 |
| 3 | CCl4+HSC+ drug serum +TGF-β1 Ab | 103.40±9.87 |
| 4 | CCl4+HSC+TGF-β1 Ab+ drug serum | 93.7796.33 |
For many years, it has been accepted that HSCs play an important role in hepatic fibrosis of chronic liver disease. Many recent studies have made clear that, in chronic liver disease, HSCs also play an important role in the regulation of intrahepatic vascular resistance and blood flow at the sinusoidal level. Accumulating evidence from in vitro and in vivo studies point to the importance both of the anatomical location of HSCs and of their capacity to contract or relax like smooth muscle cells in response to various vasoactive mediators, including endothelin-1 and nitric oxide[9-11]. It is the key to the progress of chronic liver disease, hepatic fibrosis and the elevation of intrahepatic vascular resistance. In this process what is called “activation”, HSCs get the character of myofibroblast, proliferate and synthesize ECM, however, TGF-β1 is the strongest factor to facilitate the activation of HSCs. TGF-β1 activates the static HSCs and turns them into the phenotype of myofibroblast to express α-smooth muscle actin (α-SMA) and posses the character of contraction, so as to enhance the blood resistance in liver sinuses and cause the elevation of portal vein pressure. Therefore, the activation of HSCs is the center tache in the formation of hepatocirrhosis and portal hypertension. In the course of HSCs activation, [Ca2+]i channels open widely and increase the concentration of [Ca2+]i which induces obvious contraction of cell. The increase of [Ca2+]i concentration in HSCs has the direct connection with the contraction of HSCs[12]. Activated HSCs secrete TGF-β1 and other many kinds of cell factors by self- secretion. TGF-β1 stimulates HSCs to synthesize and secrete ECM, which forms the abnormal accumulation to facilitate the activation of HSC, and contributes to the maintenance and aggravation of the hepatic fibrosis.
In this study, the technology of Flou-3/AM fluorescence probe and LSCM were used to observe the influences of anti-fibrosis I on [Ca2+]i concentration in HSCs. Flou-3 is a kind of sensitive [Ca2+]i indicator. It can combine with [Ca2+]i specifically and generate the fluorescence after certain wavelength excitation light, fluorescence spectrum changes after combination with [Ca2+]i, and there is a positive relation between the fluorescence intensity and the concentration of intracellular free [Ca2+]i. Flou-3 is an acid compound and it is difficult to enter cells, but it can enter cells through the cell membrane when Flou-3 is cultivated with cells after that Flou-3 combines with lipophylic AM to become fat-soluble Flou-3/AM, and it can turn back to Flou-3 under the effects of intracellular lipase, Flou-3 combines with intracellular free [Ca2+]i again.
Our results showed that CC14 stimulation significantly increased [Ca2+]i. By contrast, [Ca2+]i significantly decreased after anti-fibrosis I drug serum or the TGF-β1 antibody stimulation. [Ca2+]i was not significantly different between rat serum without anti-fibrosis I and untreated group. This showed rat serum itself did not affect the activation of HSC. Results of the analysis of variance for orthogonal design indicated that the different concentrations of CC14, anti-fibrosis I drug serum and the TGF-β1 antibody had great effects to the change of [Ca2+]i concentration in HSCs. CC14 increased the [Ca2+]i concentration to cause the cells injury. Activated HSCs secreted many kinds of cell factors. Inactive TGF-β1 of self-secretion was activated by the enzymes and cell factors in the circumstance of inflammation, afterward, stimulated HSCs to produce TGF-β1, in turn to make the magnified effects gradually and lead to the overproduction and activation of TGF-β1 , which made HSCs contract continuously. TGF-β1 antibody and drug serum could decrease the [Ca2+]i elevation caused by CC14. This showed that they could restrain the HSCs injury caused by CC14. If HSCs were pretreated with TGF-β1 antibody and drug serum, [Ca2+]i decreased obviously .Therefore, it was considered that TGF-β1 and drug serum could restrain the contraction of HSCs and reduce the resistance of liver sinuses by decreasing [Ca2+]i. It indicated that TGF-β1 antibody and drug serum had some effects to the treatment and prevention of hepatic fibrosis and portal vein hypertension.
Since TGF-β1 is an important factor in hepatic fibrosis, we attempted to treat hepatic fibrosis through restraining the excessive expression or abnormal modulation of TGF-β1. In order to explore the mechanism of anti-fibrosis I to prevent and treat hepatic fibrosis and portal vein hypertension from cell factors level, in this study, TGF-β1 antibody was used to block the action of TGF-β1 , interrupt the effects of some media or factors on TGF-β1 primary synthesis, decrease the effect of stimulation on ECM synthesis. TGF-β1 can facilitate activated HSC to turn into myofibroblast by self- secretion and side secretion. Activated HSCs and myofibroblasts are the major producers of ECM in liver injury, and play a prominent role in liver fibrosis. TGF-β1 can promote the progression of hepatic fibrosis with tumor necrosis factor-α (TNF-α), insulin like growth factor-1 (IGF-1), platelet derived growth factor (PDGF) etc. Anyhow, TGF-β1 can induce and facilitate hepatic fibrosis through many kinds of approaches and is considered to be a strong cell factor of fibrosis. It had been approved that TGF-β1 in the cultured HSCs of rats in vitro increase two times after addition of extrinsic TGF-β1[13]. In the progression of hepatic fibrosis, the TGF-β1 secreted by HSCs itself through self-secretion stimulation made HSC synthesize collagen more obviously. It has been found that the synthesizing and decomposing of ECM was modulated by TGF-β1 at high degree in recent few years. Animal experiment about hepatic fibrosis showed that the elevation of TGF-β1 gene expression prior to the increase of collagen synthesis, indicated that TGF-β1 had some effects to beginning of hepatic fibrosis[14]. ECM increased and HGF (hepatocyte growth factor) was restrained if TGF-β1 was added into cultured HSCs, on the contrary, ECM was restrained and HGF increased if TGF-β1 antibody was added. Rockey et al[15] found that the degree of liver injury was consistent with that of HSCs activation and contraction and the contraction of HSCs could lead to the elevation of resistance in cirrhosis liver and pressure of portal vein. One study has proved[16] that the TGF-β1 antibody could resist the biology effect of TGF-β1 at the same time, prevent HSCs from self-secreting TGF-β1 . Another study has reported[17] that monoclonal antibody of TGF-β1 could obviously restraint the proliferation of fibroblast ,and had dose-effect relationship in some range. Because of the important effect of TGF-β1 in the course of fibrosis, the use of TGF-β1 antibody might give some new clues to the explore the mechanism and therapy of fibrosis. After turning to myofibroblast, HSC itself secreted TGF, PDGF and other cell factors to make its activation go on. Even though, primary activation factors were cancelled, the progress of fibrosis would still last. It indicated that hepatic fibrosis must be treated in the early period. Because, there is mutual promoting or restraining role among cell factors, in the same cell. However, the results are different due to the difference of dosage and lasting time of cell factors. Therefore, according to the results of orthogonal trial, the best level of each factor was chosen to study the mechanism of anti-fibrosis I. After blocking the effects of TGF-β1 with TGF-β1 antibody, anti-fibrosis I drug serum decreased [Ca2+]i most significantly. If drug serum prior to TGF-β1 antibody was added into HSCs, the [Ca2+]i was higher than the former, but both were lower than [Ca2+]i in HSCs treated with drug serum and TGF-β1 antibody alone. These results suggested Anti-fibrosis I drug serum might induce HSCs relaxation by reducing [Ca2+]i in activated HSCs, and the mechanism was independent of decreasing TGF-β1 effects.
Studies have shown that [Ca2+]i is elevated either by hydrolysis of inositol lipids or by Ca2+ influx into the cell through receptor/voltage gated Ca2+ channels in the cell plasma membrane. It was reported[18] that 861 compound decreased the free [Ca2+]i to modulate the contraction and relaxation of HSCs. 861 compound depressed the expression of HSCs L-type voltage-operated calcium channel (L-VOCC), decreased the concentration of [Ca2+]i, restrained the expression of α-SMA and reduced the contraction of HSCs which might be useful to the therapy of portal vein hypertension in the early time. Yet it was considered[19]. The pharmacological mechanism of octreotide, used to treat portal hypertension with esophageal variceal bleeding, was via the relaxation of activated HSCs and the consequent decrease in intrahepatic resistance, through the activation of HSCs somatostatin receptors (SSTR), leading to a reduction in intracellular Ca2+, not interdicting the L-VOCC on the membranes of HSCs. Chinese Herbal compound can affect the construction and function of HSCs through the multi-target sites and it is important to prevent and treat hepatic fibrosis and early stage portal vein hypertension. The pharmacological mechanism of anti-fibrosis I herbal compound, used to treat hepatic fibrosis and portal hypertension, is via the relaxation of activated HSCs, and the consequent decrease in intrahepatic resistance, leading to a reduction in [Ca2+]i. Although cell factors play an important role in pathological process of hepatic fibrosis, the interaction between them is very complicated in vivo, so the potential mechanism of anti-fibrosis I will be further studied.
Edited by Guo SY Language Editor Elsevier HK
| 1. | Liu P, Liu C, Xu LM, Hu YY, Xue HM, Liu CH, Zhang ZQ. Effects of Fuzheng Huayu 319 recipe on liver fibrosis in chronic hepatitis B. World J Gastroenterol. 1998;4:348-353. [PubMed] |
| 2. | Pinzani M, Marra F, Carloni V. Signal transduction in hepatic stellate cells. Liver. 1998;18:2-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 173] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Reynaert H, Thompson MG, Thomas T, Geerts A. Hepatic stellate cells: role in microcirculation and pathophysiology of portal hypertension. Gut. 2002;50:571-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 212] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 4. | Basile DP. The transforming growth factor beta system in kidney disease and repair: recent progress and future directions. Curr Opin Nephrol Hypertens. 1999;8:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Gray MO, Long CS, Kalinyak JE, Li HT, Karliner JS. Angiotensin II stimulates cardiac myocyte hypertrophy via paracrine release of TGF-beta 1 and endothelin-1 from fibroblasts. Cardiovasc Res. 1998;40:352-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 303] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 6. | Isaka Y, Akagi Y, Ando Y, Tsujie M, Sudo T, Ohno N, Border WA, Noble NA, Kaneda Y, Hori M. Gene therapy by transforming growth factor-beta receptor-IgG Fc chimera suppressed extracellular matrix accumulation in experimental glomerulonephritis. Kidney Int. 1999;55:465-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Greenwel P, Schwartz M, Rosas M, Peyrol S, Grimaud JA, Rojkind M. Characterization of fat-storing cell lines derived from normal and CCl4-cirrhotic livers. Differences in the production of interleukin-6. Lab Invest. 1991;65:644-653. [PubMed] |
| 8. | Zou Y, Gong DZ, Sun LJ, Mei MH. Protection of hepatocyte growth factor against carbon tetrachloride injury in primary rat hepatocyte culture. Zhongguo Yiyong Shenglixue Zazhi. 1997;13:228-230. |
| 9. | Görbig MN, Ginès P, Bataller R, Nicolás JM, Garcia-Ramallo E, Cejudo P, Sancho-Bru P, Jiménez W, Arroyo V, Rodés J. Human hepatic stellate cells secrete adrenomedullin: potential autocrine factor in the regulation of cell contractility. J Hepatol. 2001;34:222-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Sakamoto M, Uen T, Nakamura T, Hashimoto O, Sakata R, Kin M, Ogata R, Kawaguch T, Torimura T, Sata M. Estrogen upregulates nitric oxide synthase expression in cultured rat hepatic sinusoidal endothelial cells. J Hepatol. 2001;34:858-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Bataller R, Ginès P, Nicolás JM, Görbig MN, Garcia-Ramallo E, Gasull X, Bosch J, Arroyo V, Rodés J. Angiotensin II induces contraction and proliferation of human hepatic stellate cells. Gastroenterology. 2000;118:1149-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 350] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 12. | Yee HF. Ca2+ and rho signaling pathways: two paths to hepatic stellate cell contraction. Hepatology. 2001;33:1007-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Weiner FR, Giambrone MA, Czaja MJ, Shah A, Annoni G, Takahashi S, Eghbali M, Zern MA. Ito-cell gene expression and collagen regulation. Hepatology. 1990;11:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 128] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Nakatsukasa H, Nagy P, Evarts RP, Hsia CC, Marsden E, Thorgeirsson SS. Cellular distribution of transforming growth factor-beta 1 and procollagen types I, III, and IV transcripts in carbon tetrachloride-induced rat liver fibrosis. J Clin Invest. 1990;85:1833-1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 272] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Rockey DC, Weisiger RA. Endothelin induced contractility of stellate cells from normal and cirrhotic rat liver: implications for regulation of portal pressure and resistance. Hepatology. 1996;24:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 255] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Eghbali-Fatourechi G, Sieck GC, Prakash YS, Maercklein P, Gores GJ, Fitzpatrick LA. Type I procollagen production and cell proliferation is mediated by transforming growth factor-beta in a model of hepatic fibrosis. Endocrinology. 1996;137:1894-1903. [PubMed] |
| 17. | Zhao Y, Tong Z, Xu L, Zhao H, Hou X. The effect of anti-transforming growth factor-beta1 antibody on fibroblast proliferation in vitro. Zhonghua JieHe He HuXi ZaZhi. 1999;22:101-103. [PubMed] |
| 18. | Shen FJ, Tang SZ, Yin CH, Wang P, Ma XM, Li J, Zhang Y, Ma H, Jia JD, Wang BE. The effect of compound 861 on hepatic stellate cell proliferation and collagen synthesis. Linchuang He Shiyan Yixue Zazhi. 2003;3:145-148. |
| 19. | Huang C, Ding HG, Xu YL, Tang SZ, Wang BE, Jia JD, Zhao CH. Effect of somatostatin on calcium concentration in hepatic stellate cells and its signification. Zhonghua Xiaohua Zazhi. 2002;22:508-509. |