Published online Mar 14, 2005. doi: 10.3748/wjg.v11.i10.1508
Revised: July 1, 2004
Accepted: July 11, 2004
Published online: March 14, 2005
AIM: To explore the changes of nuclear factor-kappa B (NF-κB) DNA-binding activity, the expression of intercellular adhesion molecule-1 (ICAM-1) regulated by NF-κB at various times and to evaluate the effects of pyrrolidine dithiocarbamate (PDTC) on trinitrobenzene sulfonic acid (TNBS)-induced rat colitis.
METHODS: TNBS of 0.6 mL was mixed with ethanol of 0.3 mL solution and instilled into the lumen of the rat colon. The rat models were divided into 6 groups, which were killed at 24 h, 3, 7, 14, and 21 d after enema. Colonic inflammation and damage were assessed by macroscopical and histological criteria. Activity of NF-κB DNA-binding was analyzed by electrophoresis mobility shift assays (EMSA). Expression of ICAM-1 was detected by in situ hybridization (ISH) and immunohistochemistry (IH). Then various doses of PDTC were injected into rat abdomen 30 min before enema with TNBS/ethanol as pretreatment. The rats were killed 4 h after enema and the colonic inflammation, myeloperoxidase (MPO) activity, malondialdehyde (MDA) level, and DNA-binding activity of NF-κB were assessed. Finally, PDTC was injected intraperitoneally after colitis was induced. Changes of morphology were assayed.
RESULTS: During the first week, hyperemia, hemorrhage, edema and ulceration of the colonic mucosa appeared with predominant infiltration of leukocytes. Neutrophils, macrophages, lymphocytes infiltrated in mucosa and submucosa 14 d later. Fibroblasts and granuloma-like structures were also obviously seen. The binding activity of NF-κB began to increase at 24 h time point and reached a peak at 14 d, then decreased but still was higher than control group at 21 d (P<0.01). Levels of ICAM-1 mRNA and protein significantly elevated at 24 h and the peak was at 21 d. Pretreatment with PDTC could attenuate the development of inflammation but not by reducing NF-κB activity. This attenuation of inflammation had a positive relationship with the dose of PDTC. PDTC at the dose of 100 mg/kg had no therapeutic effect after colitis was induced.
CONCLUSION: NF-κB activation is an important event that may be involved in acute and chronic inflammation development and may contribute to self-protection against early inflammation damage. NF-κB also regulates ICAM-1 expression during colonic inflammation. Pretreatment of PDTC may attenuate the inflammation development. But PDTC has no therapeutic effect after the colitis is induced.
- Citation: Chen K, Long YM, Wang H, Lan L, Lin ZH. Activation of nuclear factor-kappa B and effects of pyrrolidine dithiocarbamate on TNBS-induced rat colitis. World J Gastroenterol 2005; 11(10): 1508-1514
- URL: https://www.wjgnet.com/1007-9327/full/v11/i10/1508.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i10.1508
Nuclear factor-kappa B (NF-κB), which regulates inflammatory and immune processes, is a ubiquitous transcription factor. It has been proved that many inflammatory diseases, such as rheumatoid arthritis, inflammatory bowel disease and systemic inflammatory response syndrome, have the sites at which NF-κB is highly activated[1]. Once activated, NF-κB translocates to the nucleus from the cytosol, then binds to the consensus sequence on promoter or enhancer region of related gene and regulates gene transcription. It is known that several kinds of proinflammatory cytokine genes are regulated by it, such as IL-1, IL-3, IL-6, IL-8, TNF, and ICAM-1. Thus, the event of NF-κB activation has been the critical position in the inflammatory cascade. In inflammatory bowel disease, a pathogenetically unclear disease, it has been proved that NF-κB, including p50, c-Rel, and especially p65 is activated in macrophages at lamina propria. And in vitro the treatment of these cells with antisense p65 oligonucleotides reduced proinflammatory cytokine production[2]. Another research showed the administration of antisense phosphorothioate oligonucleotides to p65 subunit of NF-κB attenuated colitis in IL-10-deficient mice and in trinitrobenzene sulfonic acid-induced colitis model[3].
Intercellular adhesion molecule-1 (ICAM-1) is a cytokine-inducible glycoprotein belonging to the immunoglobulin supergene family. It is constitutively expressed on a limited number of cell types including endothelial cells and it is induced on a wide variety of cells by inflammatory cytokines such as IL-1, TNF, IFN[4]. There is a ligand by which the leukocyte integrins leukocyte function associated molecule-1 and Mac-1 and participates in leukocyte adhesion to activated endothelial cells, T cell/antigen presenting cell, T cell/T cell, and T cell/B cell interactions. So when inflammation happens, it can recruit leukocytes to local inflammatory region resulting in acceleration and development of disease. Van der Saag found that there was a specific ICAM-1 gene sequence (TGGAAATTCC) bound to NF-κB and its transcription was regulated by activated NF-κB[5].
Pyrrolidine dithiocarbamate (PDTC) represents a kind of antioxidants reported to be an effective NF-κB inhibitor through its ability to traverse the cell membrane and its prolonged stability in solution at physiological pH. The report by Cuzzocrea showed that it could attenuate acute and chronic inflammatory development induced by activated NF-κB[6].
The aims of this study were two-fold. First, to establish a colonic inflammatory rat model similar to human Crohn’s disease by the intraluminal instillation of a solution containing 2,4, 6-trinitrobenzenesulfonic acid/ethanol and explore the significance of DNA-binding activity changes of NF-κB at various times in this model. Meanwhile, to study the expression of ICAM-1 regulated by NF-κB in colonic tissue. Second, to evaluate the effects (including pretreatment and therapy effects) of pyrrolidine dithiocarbamate (PDTC) on acute and chronic colitis.
2,4,6-Trinitrobenzenesulfonic acid 5% was purchased from Sigma (USA). EMSA kit was from Promega (USA). Occult blood test reagents, myeloperoxidase (MPO) and malondialdehyde (MDA) reagent kits were provided by Nanjing Jiancheng Biological Research Unit (China). γ-32PATP was purchased from Beijing Furui Company (China). In situ hybridization (ISH) kit, immunohistochemistry (IH) kit of ICAM-1 and anti-rabbit polyclonal antibody of ICAM-1 was from Wuhan Boshide Biological Company (China). Anti-rat monoclonal antibody of p65 was from Beijing Zhongshan Biological Company (China). All other chemicals were obtained from either Sigma or Beijing Dingguo Company. Male Sprague-Dawley (S-D) rats (170-200 g body wt) were from specific pathogen free unit in Animal Center of Guangdong Medical College.
All rats were maintained at 23 °C on a 12 h light/dark cycle and allowed free access to water and standard laboratory chow. From 12 h before the start of the experiments, the animals were deprived of food except access to water.
For NF-κB activity study, rats were divided into six groups by randomized block design: (1) enema with 0.9 mL normal saline (NS group, n = 6, killed at 24 h), (2) enema with 0.6 mL TNBS+0.3 mL ethanol once (T/E group, n = 30, killed at 24 h, 3, 7, 14, 21 d independently). The macroscopic and microscopic damage score criteria were according to Butzner’s and Wang’s[7,8].
For the PDTC pretreatment study, 48 rats were divided into six groups: (1) enema with normal saline (N group, n = 8), (2) enema with TNBS/ethanol and normal saline given as an intraperitoneal (i.p.) bolus 30 min before enema (M group, n = 8), (3) various doses of PDTC, respectively 10, 25, 50, 100 mg/kg, were injected into rat abdomen 30 min before enema (P10, P25, P50, P100 groups, n = 32). Rats were bled to death 4 h after enema. The microscopic damage score criteria were according to Millar’s[9].
For the PDTC therapy study, 30 rats were divided into three groups: (1) enema with normal saline and normal saline given i.p. every 48 h starting from d 1 (group A, n = 10), (2) enema with TNBS/ethanol and normal saline given i.p. starting from d 1 (group B, n = 10), (3) enema with TNBS/ethanol and PDTC given (100 mg/kg) i.p. starting from d 1 (group C, n = 10). Rats were killed on d 14. The morphology damage score was according to Butzner’s[7].
The colon was removed, freed from surrounding tissues, opened along the antimesenteric border, rinsed, and weighed. One part was processed for histology, immunohistochemistry and in situ hybridization. The other part was stored in liquid nitrogen for NF-κB, MPO, and MDA detection.
Nuclear protein was extracted as described by Gukovsky et al[10]. Briefly, colon tissue was pounded to pieces in liquid nitrogen, then homogenized in 312 μL of cold buffer A (10 mmol/L HEPES-KOH, 1.5 mol/L MgCl2, 10 mmol/L KCl, 1 mmol/L PMSF, 1 mmol/L DTT, 10 μg/mL leupeptin, 35 μg/mL aprotinin) for 10-20 times. The samples were separated by centrifugation (0 °C, 2000 r/min, 15 s) and the supernatant was incubated in ice water for 5 min, and then spun by centrifugation (0 °C, 5000 r/min, 10 s) again. The supernatant was discarded and 104 μL buffer B (20 mmol/L HEPES-KOH, 25% glycerol, 420 mmol/L NaCl, 1.5 mmol/L MgCl2, 1 mmol/L PMSF, 1 mmol/L DTT, 10 μg/mL leupeptin, 35 μg/mL pepstatin, 10 μg/mL aprotinin) was added. The deposits were incubated in ice water for 20 min. Finally, the samples were separated by centrifugation (0 °C, 12000 g, 15 min). Supernatant liquid was stored at -70 °C. Protein concentrations were determined by Bradford assay.
The activity of NF-κB DNA-binding was detected by EMSA as described by Molloy with the following modification[11]. The double-stranded oligonucleotide sequence of 5’-AGTTGAGGGGACTTTCCCAGGC-3, 3’-TCAACTC-CCCTGAAAGGGTCCG-5’, which corresponds to the κB binding site, was end-labeled with [γ-32P]ATP by T4 polynucleotide kinase (Promega). Labeled probes were purified and counts per minute (CPM) were detected with Whatman DE81 (>5000 CPM). The nuclear extract equivalent to 15 μg was mixed with 5×binding buffer 2 μL (room temperature, 10 min), then labeled probe (0.0175 pmoL) was added and incubated (room temperature, 20 min). The mixture was then subjected to electrophoresis on 4% polyacrylamide gel at 150 V in 0.5×TBE buffer for 40 min at 4 °C. After being dried, the gel was exposed to imaging system of FX P screen (Bio-Rad, America) for 24 h. The result was analyzed with software Bandscan 4.0 (denary logarithm of total grey value). Specific competition was performed by addition of specific unlabeled double-stranded oligonucleotide to the reaction mixture in 50-100 fold excess, while nonspecific competition with AP2 oligonucleotide. Supershift assay was performed with the rabbit monoclonal antibody against p65 to verify p65 subunit.
In situ hybridization (ISH) and immunohistochemistry (IH) were employed to detect ICAM-1 mRNA and protein, as described from reagent kit. Immunohistochemical score (IHS) was applied to evaluate ISH or IH described by Soslow[12]. IHS was calculated by combining an estimate of the percentage of immunoreactive cells (quantity score) with an estimate of the staining intensity (staining intensity score), as follows. 0: no staining; 1: 1-10% of cells stained; 2: 11-50% of cells stained; 3: 51-80% of cells stained; 4: 81-100% of cells stained. Staining intensity was rated on a scale of 0-3, with 0 = negative; 1 = weak; 2 = moderate, and 3 = strong. When there was multifocal immunoreactivity and there are significant differences in staining intensities between foci, the average of the least intense and most intense staining was recorded. The raw data were converted to IHS by multiplying the quantity and staining intensity scores.
MPO, T-SOD and MDA were determined by the methods of the respective kits.
Parametric data were presented as mean+SD and statistical analyses were performed using the two-way ANOVA or t-test with the software package SPSS 8.0. Results were considered significant at a P value of less than 0.05.
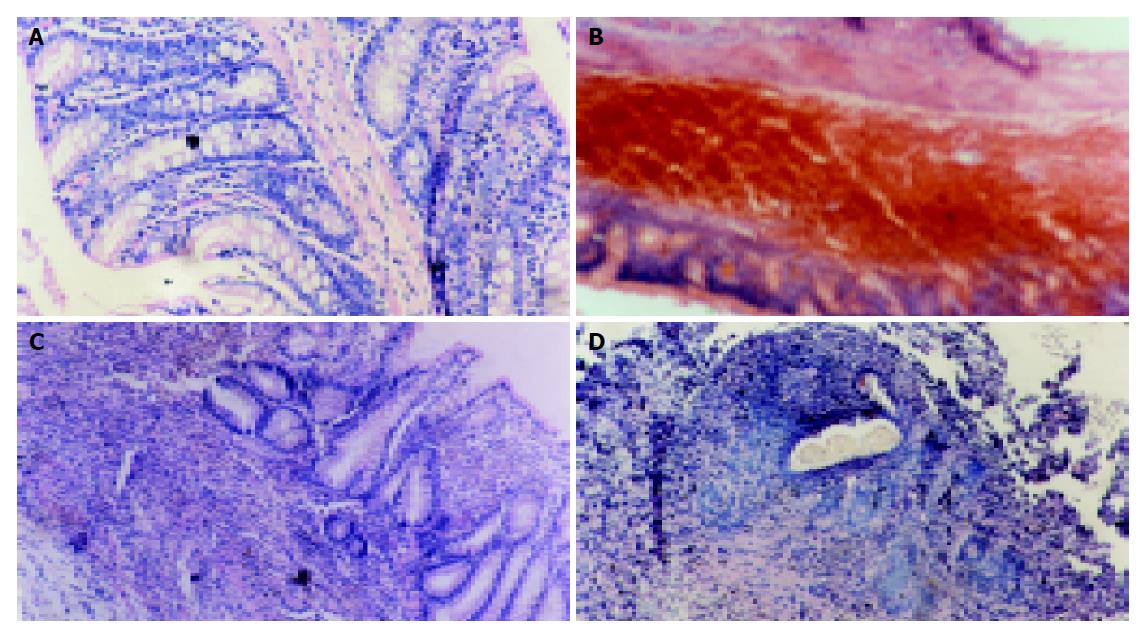
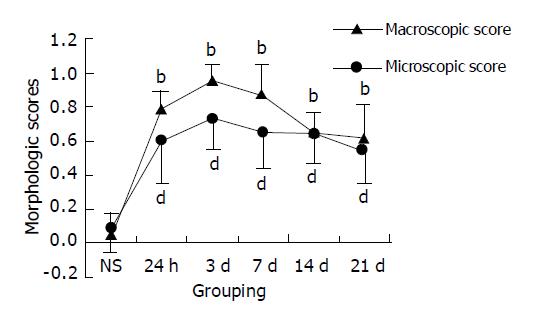
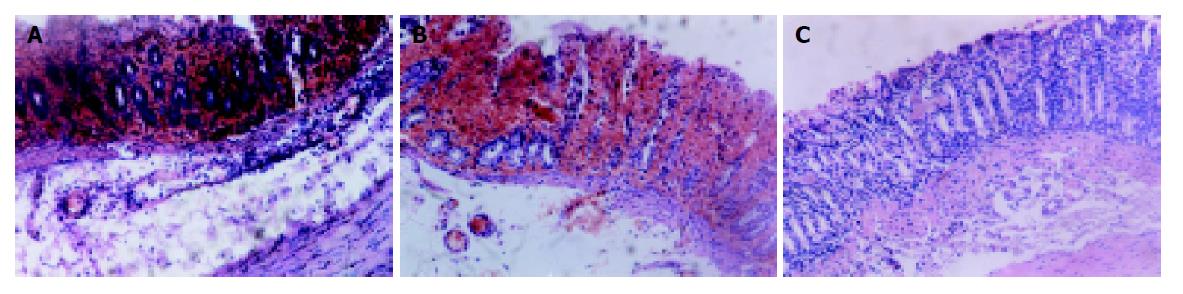
Intracolonic administration of TNBS/ethanol to S-D rats induced a severe illness characterised by bloody diarrhea and a dramatic loss of body weight during the first week. Then the body weight increased but diarrhea still persisted for about two weeks. Two rats died on d 5, 12. No changes could be observed in NS group. Macroscopic evaluation of the colon and rectum up to 24 h after TNBS treatment revealed the presence of mucosal edema and hemorrhagic ulcerations. Conglutination was obvious later. Histologic examination showed predominant infiltration of leukocytes and erythrocytes in mucosa and submucosa in the first week. Neutrophils, macrophages, lymphocytes infiltrated in mucosa, submucosa and muscularis propria 14 d later (Figures 1A-D). Fibroblasts and granuloma-like structures were also obviously seen. No damage was seen in NS group. The macroscopic and microscopic damage score showed the peak at d 3, then decreased (scores in T/E group at various time points vs scores in NS group, P<0.01) (Figure 2).
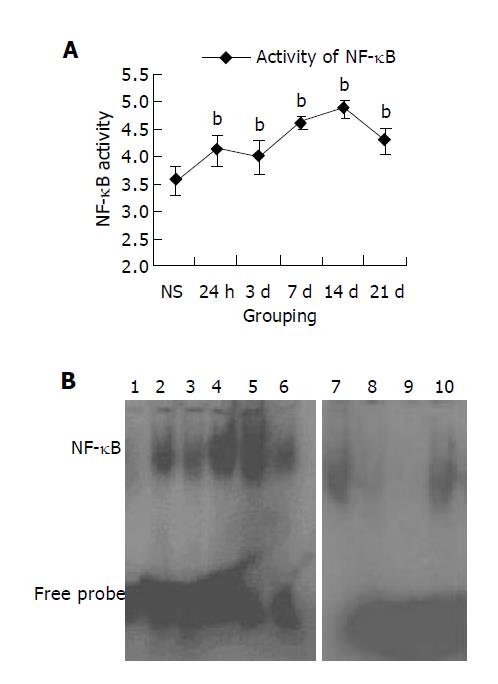
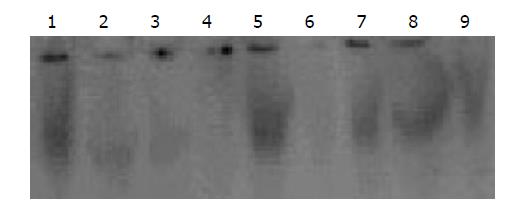
Nuclear extracts from normal or inflamed colon tissue at various time points were isolated and analyzed by EMSA. The EMSA analysis showed a strong activation of NF-κB in rat inflamed colon tissue induced by TNBS/ethanol from 24 h to 21 d. It began to increase sharply at 24 h and the peak was on 14 d. The activity of NF-κB was very weak in noninflamed colon tissue of NS group. To assess the specificity of the observed DNA-protein, specific and nonspecific competition were performed by addition of unlabeled double-stranded oligonucleotide or AP2 oligonucleotide to the reaction mixture in 50-100 fold excess (Figure 3). Supershift analysis showed that pre-incubation with the antibodies against p65 was followed by a weakening and lagging of the NF-κB -DNA complex band. Almost no effect could be seen with the nonspecific antiserum. This indicates that the majority of the dimers in inflammatory intestinal tissue contained a p65 subunit.
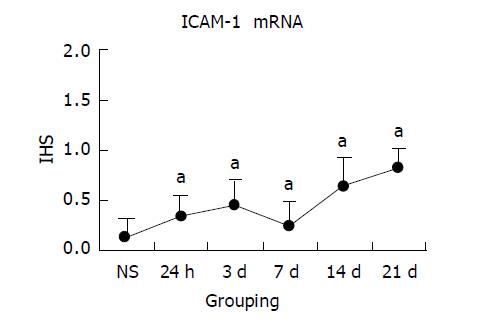
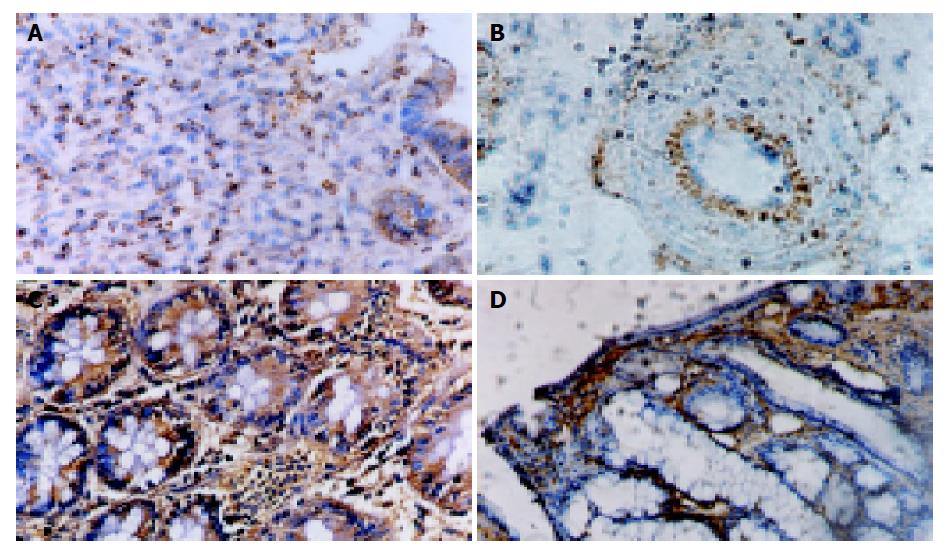
NS group colon tissue had no or weak ICAM-1 mRNA detected by ISH in endothelial or epithelial cells. Similar to NF-κB activity, ICAM-1 mRNA increased with inflammation progression in experimental groups. Following 24 h of TNBS/ethanol treatment, ISH showed that expression of ICAM-1 mRNA increased, predominantly associated with endothelial cells. And inflammatory cells also had weak expression in the lamina propria. From the IHS, the maximum of ICAM-1 expression was on 21 d, mainly in either epithelial cells or inflammatory cells from mucosa to submucosa layer. The correlation analysis between ICAM-1 mRNA score and NF-κB activity indicated that they had significant positive correlation (r = 0.913, P<0.05). Colonic ICAM-1 protein was elevated over control group in quantity and intensity after the treatment with TNBS/ethanol. Relatively high ICAM-1 expression occurred at 14 or 21 d, which was stronger in lamina propria than in submucosa (Figures 4 and 5).
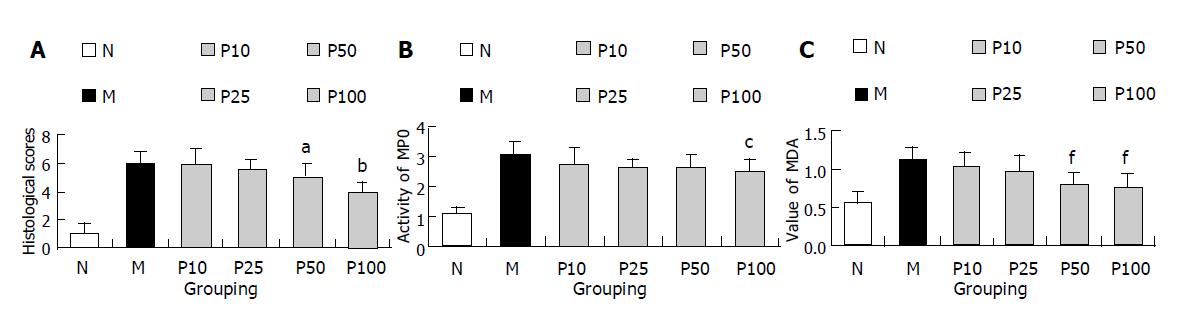
All rats treated with TNBS/ethanol developed acute colitis characterized by the erosion, edema and bleeding at mucosa and submucosa layers 4 h after TNBS/ethanol administration. It induced a significant increase in the activity of MPO and the level of MDA in colonic tissue. The level of NF-κB activity was also significantly up-regulated in colonic tissue. Pretreatment of rats with PDTC attenuated the inflammatory degree (histological score according to Millar’s criteria[9]) in a dose-related fashion. Similarly, pretreatment of rats with PDTC significantly reduced both activity of MPO and level of MDA in a dose-dependent fashion (Figures 6A-C, and 7). EMSA analysis of colonic tissue obtained 4 h after TNBS/ethanol administration showed that PDTC almost blocked NF-κB activity at the dose of 10 mg/kg but not completely reduced histological score at the same time. And the added dose of PDTC could attenuate colitis development, consequently MPO activity and MDA level decreased. Interestingly, the added dose of PDTC did not further weaken NF-κB activity but its effect was stronger than that at 10 mg/kg dose (Figure 8).
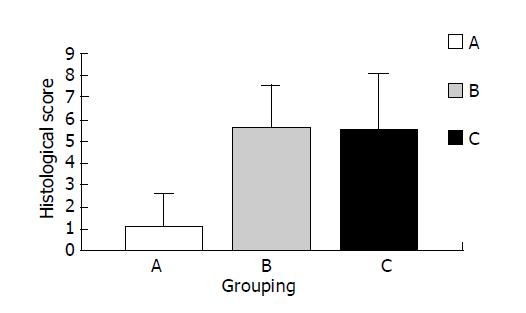
All rats treated with TNBS/ethanol developed a chronic colitis at 14 d as described above (Figure 1C, D). Both the clinical signs and morphological features of rats were observed in groups A-C. But in PDTC-treated (at the dose of 100 mg/kg) group, there was no significant change in morphology compared with B group (group A vs group B, P>0.05). (Figure 9).
Animal colitis which is similar to human Crohn’s disease can be induced experimentally in rats, mice, rabbits, pigs, and dogs by TNBS/ethanol introduced via the enema[13-18]. Trinitrobenzene sulfonic acid is a haptenating agent that couples trinitrophenyl groups to endogenous (self) proteins more or less indiscriminately; the altered self-proteins stimulate a local immunologic response usually called a delayed hypersensitivity reaction. In the colon, this reaction leads to an inflammation marked by a transmural cellular infiltration with T cells and macrophages in a pattern like that described in interleukin-2 knockout mice and Crohn’s disease[19]. Here we induced the model by 5% 0.6 mL TNBS and 0.3 mL ethanol, rats showed clinical symptoms including diarrhea, occult blood stool, and gross bleeding. Postmortem investigations of both acute and chronic colitis showed that inflammatory changes and multiple erosions appeared in whole colonic walls and were particularly prominent at submucosa layer. Hyperemia, hemorrhage, edema, and ulceration of the colonic mucosa were accompanied with predominant infiltration of leukocytes. Neutrophils, macrophages, lymphocytes infiltrated in mucosa and submucosa. Fibroblasts and granuloma-like structures were also obviously seen. This was consistent with the typical model induced by Morris[20].
As a nuclear protein, NF-κB is a eukaryotic transcription factor which was first described as a B-cell specific factor that binds to the motif in the immunoglobulin light-chain intronic enhancer. More and more evidence has confirmed that expression of many cytokines, chemokines, immunoreceptors, adhesion molecules, antiapoptotic/proapoptotic, and transcription factors were regulated by NF-κB. Once activated, the nuclear localization signal of NF-κB is uncovered and NF-κB translocates to the nucleus of the cell from the cytosol to regulate above protein expression. Inflammatory bowel diseases are characterized by the recruitment of humoral and cellular components of the immune system to certain areas of the gut. Excessive cytokines are produced by activated epithelial cells, macrophages, and B and T cells. This process is characterized by NF-κB induction. As a result, a perpetuation of the inflammatory reaction was amplified[21]. Constitutively activated NF-κB was identified in epithelial cells and lamina propria macrophages from biopsy specimens or cultured cells of patients with inflammatory bowel disease. Further, p65 is the main member closely related to the inflammatory process[22-25]. Recently it was shown that the administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-κB abrogates colitis in the trinitrobenzene sulfonic acid-induced colitis model[3]. In our study, we found NF-κB was activated in TNBS-induced rat early colitis and the binding activity of NF-κB increased with inflammatory progress in experimental groups at various time points. This result is accorded with the viewpoint that NF-κB is activated constitutively in IBD. But it did not have significant correlation with the morphology score. Furthermore, we injected PDTC at the dose of 10 mg/kg into rat abdomen 30 min before enema, which almost inhibited NF-κB DNA-binding activity. As a result, it could not block colonic inflammation development. The reason is not clear. Maybe activation of NF-κB contributes to self-protection against early inflammation damages[26].
Leukocytes and endothelial adhesion molecules play a key role in mediating the recruitment of leukocytes to inflammatory site. As a member of immunoglobulin-like, ICAM-1 expresses on endothelial cells, fibroblasts and many other cell types. Evidence has proved that ICAM-1 was up-regulated in inflammatory colonic tissue induced by TNBS[27-29]. We also found that ICAM-1 mRNA and protein expressions were increased in endothelial, epithelial cells, and inflammatory cells, such as neutrophilic, leukocyte monocytes, lympholeukocyte, with inflammatory progress in TNBS-induced groups. The scores of ICAM-1 mRNA had a significant correlation with NF-κB activity (r = 0.913, P<0.05) indicating its expression was regulated by NF-κB. Similar to NF-κB, ICAM-1 scores had no significant correlation with histological score, suggesting that ICAM-1 might be more effective on chronic colitis related to immunity than on nonspecific acute colitis. Further studies are needed.
PDTC attenuated the development of acute and chronic inflammation of pleurisy and arthritis in mice model, which had been reported by Cuzzocrea and his colleagues. The mechanism that PDTC protects against inflammatory injury is that it can inhibit the activation of NF-κB[6]. In our study, although we showed pretreatment of rats with PDTC attenuated the inflammatory degree including decrease of histological score, MPO activity, and MDA level in a dose-dependent fashion, we did not observe its anti-inflammation effect by blocking NF-κB of activity, because there was no significant effect on inflammation at the dose of 10 mg/kg, while it markedly reduced NF-κB activity. So other mechanisms of PDTC must be considered. Satriano found that PDTC had the effect to interfere with reactive oxygen metabolism[30]. When we detected the MPO activity and MDA level which indicate oxidative reaction indirectly, we also found PDTC reduced their level in a dose-related fashion. Furthermore, the chelation of divalent metal ions and influence on intracellular thiol levels also were considered[6]. Interestingly, PDTC at the dose of 100 mg/kg had no therapeutic effect when we injected it into rat abdomen every 48 h after inducing the model. By different doses, different animal models, and different vehicles, our result did not accord with Cuzzocrea’s. The reason for this is not clear.
In conclusion, NF-κB activation is an important event that may be involved in acute and chronic inflammation development and contribute to self-protection against early inflammation damage. NF-κB also regulates ICAM-1 expression during colonic inflammation and pretreatment of PDTC may attenuate the inflammatory development but has no therapeutic effect on induced colitis.
Edited by Zhu LH Language Editor Elsevier HK
| 1. | Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3011] [Cited by in RCA: 3176] [Article Influence: 132.3] [Reference Citation Analysis (0)] |
| 2. | Neurath MF, Fuss I, Schürmann G, Pettersson S, Arnold K, Müller-Lobeck H, Strober W, Herfarth C, Büschenfelde KH. Cytokine gene transcription by NF-kappa B family members in patients with inflammatory bowel disease. Ann N Y Acad Sci. 1998;859:149-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 172] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Neurath MF, Pettersson S, Meyer zum Büschenfelde KH, Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med. 1996;2:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 631] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 4. | Jones SC, Banks RE, Haidar A, Gearing AJ, Hemingway IK, Ibbotson SH, Dixon MF, Axon AT. Adhesion molecules in inflammatory bowel disease. Gut. 1995;36:724-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 150] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | van der Saag PT, Caldenhoven E, van de Stolpe A. Molecular mechanisms of steroid action: a novel type of cross-talk between glucocorticoids and NF-kappa B transcription factors. Eur Respir J Suppl. 1996;22:146s-153s. [PubMed] |
| 6. | Cuzzocrea S, Chatterjee PK, Mazzon E, Dugo L, Serraino I, Britti D, Mazzullo G, Caputi AP, Thiemermann C. Pyrrolidine dithiocarbamate attenuates the development of acute and chronic inflammation. Br J Pharmacol. 2002;135:496-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 175] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Butzner JD, Parmar R, Bell CJ, Dalal V. Butyrate enema therapy stimulates mucosal repair in experimental colitis in the rat. Gut. 1996;38:568-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 155] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Wang H, Ouyang Q, Hu RW. Establishment of rat colitis model by trinitrobenzensulfonic acid. Weichang Bingxue. 2001;6:7-10. |
| 9. | Millar AD, Rampton DS, Chander CL, Claxson AW, Blades S, Coumbe A, Panetta J, Morris CJ, Blake DR. Evaluating the antioxidant potential of new treatments for inflammatory bowel disease using a rat model of colitis. Gut. 1996;39:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 220] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Gukovsky I, Gukovskaya AS, Blinman TA, Zaninovic V, Pandol SJ. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am J Physiol. 1998;275:G1402-G1414. [PubMed] |
| 11. | Molloy PL. Electrophoretic mobility shift assays. Methods Mol Biol. 2000;130:235-246. [PubMed] |
| 12. | Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, Koki AT. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89:2637-2645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 13. | Hoffmann JC, Peters K, Henschke S, Herrmann B, Pfister K, Westermann J, Zeitz M. Role of T lymphocytes in rat 2,4,6-trinitrobenzene sulphonic acid (TNBS) induced colitis: increased mortality after gammadelta T cell depletion and no effect of alphabeta T cell depletion. Gut. 2001;48:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Keates AC, Castagliuolo I, Cruickshank WW, Qiu B, Arseneau KO, Brazer W, Kelly CP. Interleukin 16 is up-regulated in Crohn's disease and participates in TNBS colitis in mice. Gastroenterology. 2000;119:972-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Camoglio L, te Velde AA, de Boer A, ten Kate FJ, Kopf M, van Deventer SJ. Hapten-induced colitis associated with maintained Th1 and inflammatory responses in IFN-gamma receptor-deficient mice. Eur J Immunol. 2000;30:1486-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Linden DR, Chen JX, Gershon MD, Sharkey KA, Mawe GM. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G207-G216. [PubMed] |
| 17. | Depoortere I, Thijs T, Peeters TL. Generalized loss of inhibitory innervation reverses serotonergic inhibition into excitation in a rabbit model of TNBS-colitis. Br J Pharmacol. 2002;135:2011-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Shibata Y, Taruishi M, Ashida T. Experimental ileitis in dogs and colitis in rats with trinitrobenzene sulfonic acid--colonoscopic and histopathologic studies. Gastroenterol Jpn. 1993;28:518-527. [PubMed] |
| 19. | Strober W, Lúdvíksson BR, Fuss IJ. The pathogenesis of mucosal inflammation in murine models of inflammatory bowel disease and Crohn disease. Ann Intern Med. 1998;128:848-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795-803. [PubMed] |
| 21. | Schottelius AJ, Baldwin AS. A role for transcription factor NF-kappa B in intestinal inflammation. Int J Colorectal Dis. 1999;14:18-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 146] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Rogler G, Brand K, Vogl D, Page S, Hofmeister R, Andus T, Knuechel R, Baeuerle PA, Schölmerich J, Gross V. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115:357-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 540] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 23. | Jobin C, Haskill S, Mayer L, Panja A, Sartor RB. Evidence for altered regulation of I kappa B alpha degradation in human colonic epithelial cells. J Immunol. 1997;158:226-234. [PubMed] |
| 24. | Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998;42:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 580] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 25. | Cui HH, Chen CL, Wang JD, Yang YJ, Cun Y, Wu JB, Liu YH, Dan HL, Jian YT, Chen XQ. Effects of probiotic on intestinal mucosa of patients with ulcerative colitis. World J Gastroenterol. 2004;10:1521-1525. [PubMed] |
| 26. | Steinle AU, Weidenbach H, Wagner M, Adler G, Schmid RM. NF-kappaB/Rel activation in cerulein pancreatitis. Gastroenterology. 1999;116:420-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 196] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 27. | Maric I, Poljak L, Zoricic S, Bobinac D, Bosukonda D, Sampath KT, Vukicevic S. Bone morphogenetic protein-7 reduces the severity of colon tissue damage and accelerates the healing of inflammatory bowel disease in rats. J Cell Physiol. 2003;196:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Cuzzocrea S, Mazzon E, Dugo L, Caputi AP, Riley DP, Salvemini D. Protective effects of M40403, a superoxide dismutase mimetic, in a rodent model of colitis. Eur J Pharmacol. 2001;432:79-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Sun FF, Lai PS, Yue G, Yin K, Nagele RG, Tong DM, Krzesicki RF, Chin JE, Wong PY. Pattern of cytokine and adhesion molecule mRNA in hapten-induced relapsing colon inflammation in the rat. Inflammation. 2001;25:33-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Satriano J, Schlondorff D. Activation and attenuation of transcription factor NF-kB in mouse glomerular mesangial cells in response to tumor necrosis factor-alpha, immunoglobulin G, and adenosine 3': 5'-cyclic monophosphate. Evidence for involvement of reactive oxygen species. J Clin Invest. 1994;94:1629-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 150] [Article Influence: 4.8] [Reference Citation Analysis (0)] |