Published online Mar 14, 2005. doi: 10.3748/wjg.v11.i10.1433
Revised: September 20, 2004
Accepted: October 8, 2004
Published online: March 14, 2005
AIM: To determine the effectiveness of pre-liver transplant (LT) transarterial embolization (TAE) in treating hepatocellular carcinoma (HCC) and the patient categories, which are likely to have a good outcome after LT.
METHODS: Twenty-nine patients with hepatitis-related cirrhosis and unresectable HCC after LT were studied over a 7-year period. The patients were divided into two groups: group A patients (19/29) received pre-LT TAE, whereas group B (10/29) underwent LT without prior TAE. According to Milan criteria, group A patients were further subdivided into: group A1 (12/19) who met the criteria, and group A2 (7/19) who did not. Patient survivals were compared.
RESULTS: In the explanted liver, CT images correlated well with pathological specimens showing that TAE induced massive tumor necrosis (>85%) in 63.1% of patients in group A and all 7 patients in group A2 exhibited tumor downgrading that met Milan criteria. The overall 5-year actuarial survival rate was 80.6%. The TAE group had a better survival (84% at 5 years) than the non-TAE (75% at 4 years). The 3-year survival of group A2 (83%) was also higher than that of group A1 (79%). Tumor necrosis >85% was associated with excellent survival of 100% at 3 years, which was significantly better than the others who showed <85% tumor necrosis (57.1% at 3 years) or who did not have TAE (75% at 3 years).
CONCLUSION: TAE is an effective treatment for HCC before LT. Excellent long-term survival was achieved in patients that did not fit Milan criteria. Our results broadened and redefined the selection policy for LT among patients with HCC. Meticulous pre-LT TAE helps in further reducing the rate of dropout from waiting lists and should be considered for patients with advanced HCC.
- Citation: Cheng YF, Huang TL, Chen TY, Chen YS, Wang CC, Hsu SL, Tsang LLC, Sun PL, Chiu KW, Jawan B, Eng HL, Chen CL. Impact of pre-operative transarterial embolization on the treatment of hepatocellular carcinoma with liver transplantation. World J Gastroenterol 2005; 11(10): 1433-1438
- URL: https://www.wjgnet.com/1007-9327/full/v11/i10/1433.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i10.1433
Hepatocellular carcinoma (HCC) is the most common form of primary liver cancer worldwide and has been the leading cause of cancer death in Taiwan in recent years. HCC caused by the current epidemic of hepatitis B virus-related cirrhosis claims the lives of 5000 people each year in Taiwan. The number of new cases is still steadily increasing[1]. For patients with early disease, primary treatment is surgical resection whenever possible. Unfortunately, in patients with large and multiple tumors at the time of initial presentation, surgery is not feasible and their overall survival is usually less than 6 mo[2]. With the advance of surgical techniques in the past few years, LT is now commonly accepted as the optimal therapeutic measure because not only does it remove the cancer, but it also treats the underlying disease with eradication of the cirrhotic tissue that may progress to dysplastic nodules or HCC in the future[3]. The current United Network of Organ Sharing (UNOS) policy for organ allocation among patients with HCCs favors those potential recipients with limited number and diameter of tumor nodule defined by Milan criteria: (A) solitary tumor <5 cm, or B) three or less lesions, none of them >3 cm[4]. LT can therefore be offered with a good chance of success to only a relatively small proportion of patients, and there is a need for associated treatment regimens to improve the operation rate and to diminish the incidence of recurrence after transplantation.
Various non-surgical therapeutic options for advanced HCC have been introduced, including TAE, percutaneous ethanol injection, systemic chemotherapy, hormone therapy, immunotherapy, and radiotherapy, among which TAE plays the major role as a widely accepted treatment[5]. Transarterial embolization (TAE) is a procedure involving the injection of lipiodol and chemotherapeutic agent into the hepatic artery, followed by embolization with absorbable gelatin particles. It produces a selective ischemic and pharmacologic injury to the tumor that relies mainly on the arterial circulation. TAE was first introduced as a palliative treatment for patients with inoperable disease and achieved good results. In the past few years, the concept of blocking collateral blood supply to the tumor through complete embolization of liver tissue surrounding the tumor to achieve curative treatment for hepatic malignancies has been proposed. Moreover, the transarterial administration of a mixture of lipiodol and ethanol to create dual hepatic arterial and portal venous embolization to attain the effect of lobar ablation has been documented[6]. More importantly, TAE has also been applied to improve the resectability of primary unresectable tumors[7] because it effectively decreases tumor size, causes compensatory hepatic hypertrophy, and improves ICGR15 that allows a wider range of patients to undergo liver surgery with the achievement of a better survival. Pre-transplant adjuvant treatments, therefore, plays an important role in reducing the dropout rate of the waiting list for LT. Hence, not only is TAE the treatment of choice for unresectable HCC to induce tumor necrosis and to control tumor progression, it may also be beneficial for enlisted patients for LT while waiting for the suitable grafts. The aim of this study is to evaluate the effect of pre-transplantation TAE on patients with HCC.
Patients with histologically proven HCC or a clinical and radiological presentation strongly suggestive of HCC were considered for the protocol. All were deemed unresectable, either because of anatomic considerations or inadequacy of hepatic reserve. The absence of metastatic tumor was documented with computed tomography (CT) of the chest, abdomen, and pelvis. Tumor invasion of the portal vein was assessed with ultrasound, CT angiography and magnetic resonance scans. Invasion to portal vein was an exclusion criterion. If the patients fully fit the Milan criteria and liver graft was available, then LT proceeded. Otherwise, the patients were included into the TAE group. TAE was performed in the absence of contraindications and poor liver function in the Child’s class C. If the TAE was well tolerated, it was repeated if necessary until a donor organ became available.
All patients received complete celiac and superior mesenteric artery injection for the localization of hepatomas in the liver before embolization. The 4 F catheter was advanced into the feeding artery as distally as possible. Throughout this study, coaxial 3F catheter was used in all patients. By using syringe pump (Razel Scientific Instruments Inc., Stamford, CT) which can control the injection rate ranging from 0.1 to 1.2 mL/min, a mixture of iodized oil/ethanol (99.5%) in the ratio of 2:1 was infused selectively into the supplying artery at a flow rate of 0.5 to 1 mL/min until the adjacent portal branches of the segmental or lobar liver were demonstrated. The process was under remote manual fluoroscopic guidance outside the angiographic room. The results of embolization were evaluated by CT in all patients 2 wk after the procedure. We classified the results as complete if lipiodol occupied the whole tumor (100%), above 85% as partial embolization, 85% or below as incomplete embolization. All cases with partial or incomplete embolization received second embolization 3 to 4 wk later after liver function was resumed. Following radiological restaging after TAE, the patients underwent liver transplantation when a graft became available either from a cadaveric or a living donor. The discovery of extrahepatic tumor either during radiological staging or at laparotomy precluded LT.
The explanted liver specimens were examined for features of tumor disease, including the size, number of nodules, presence of portal vein thrombosis and percentage of tumor necrosis. The tumor size and number were also measured on the pre-TAE/LT sonography and CT. The size and number of the tumor on the explanted liver were taken as the basis for staging to be compared with Milan criteria. Downgrading was defined as the size and number of the tumors in the explanted liver fully fit the criteria: A) solitary tumor <5 cm or B) ≤3 lesions none of them>3 cm, but the initial pretreatment images exceeded these criteria.
Immunosuppressive therapy after LT consisted of a triple drug regimen of tacrolimus, corticosteroids, and either azathioprine or mycophenolate mofetil. Corticosteroids were gradually tapered and were discontinued in 3 mo. All patients were followed up weekly in the outpatient clinic in the first few months after discharge. The frequency of the outpatient clinic visits thereafter varied according to the patients’ conditions and types of complications. Screening for tumor recurrence was assessed by the measurement of serum alpha fetal protein (AFP) and abdominal sonography every 2-3 mo. CT scans of the abdomen and chest were performed if HCC recurrence was suspected.
The biomedical statistical program Statistica 4.0 (Statsoft, Tulsa, OK) was used for statistical analysis where appropriate. The Kaplan-Meier method was used to calculate survival and groups were compared with the log-rank test. P value less than 0.05 was considered significant.
In the 8-year period from 1996 to 2003, 29 patients in our program underwent LT treatment for histologically confirmed HCC associated with cirrhosis. There are 28 male and 1 female with age of 50.03±8.93 years (mean±SD, range: 24-67). The nature of underlying liver cirrhosis was hepatitis B in 21 (HBsAg positive), hepatitis C (determined by HCV RNA testing) in 7, and combined hepatitis B and C in 1.
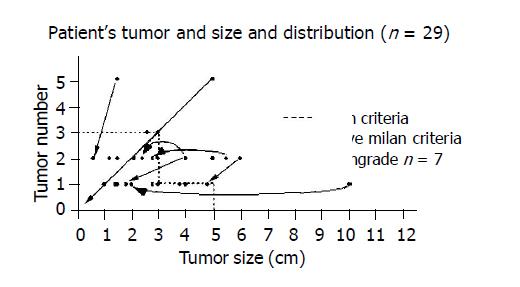
In group A, 19 patients (19 males and 0 female, age: 52.4±7.61 years) with sufficient hepatic function underwent TAE in the treatment of HCC before LT. Of these 19 patients, 12 met the Milan criteria (group A1) and 7 exceeded the criteria (group A2). In group B, 10 patients (9 males and 1 female, age: 45.5±9.89 years) received LT without prior TAE because of available liver graft. Of these 10 patients who met the criteria for transplant, 4 had inadequate liver function for TAE. The mean waiting time from diagnosis to LT was 19.7±18.2 mo in the TAE group and 12.6±12.7 mo in the non-TAE group (Table 1 and Figure 1).
| TAE (group A, %) | No TAE (group B, %) | P | |
| Number of patient | 19 (66) | 10 (34) | |
| Age (yr) (mean±SD) | 52.4±7.61 | 45.5±9.89 | 0.045 |
| Follow-up time (d) | 769±395 | 708±403 | 0.697 |
| Time from diagnosis to | 19.7±18.2 | 12.6±12.7 | 0.281 |
| liver transplant | |||
| Male/Female | 19 (100)/0 (0) | 9 (90)/1 (10) | 0.345 |
| Hepatitis virus | 0.787 | ||
| Hepatitis B | 14 (74) | 7 (70) | |
| Hepatitis C | 4 (21) | 3 (30) | |
| Hepatitis B & C | 1 (5) | 0 (0) | |
| Initial tumor size (cm) | 0.597 | ||
| < = 3 cm (number of patient) | 12 (63) | 8 (80) | |
| >3, < = 5 cm | 4 (21) | 2 (20) | |
| >5 cm | 3 (16) | 0 (0) | |
| Tumor number | 0.481 | ||
| 1 nodule | 9 (47) | 7 (70) | |
| 2 nodule | 7 (37) | 2 (20) | |
| 3 nodule | 1 (5) | 1 (10) | |
| >3 nodule | 2 (11) | 0 | |
| TAE (number of courses) | |||
| 1 (number of patient) | 10 (53) | -- | |
| 2 | 3 (16) | -- | |
| >2 | 6 (32) | -- | |
| Above Milan criteria before TAE or transplantation | 71 (group A2) | -- |
The explanted liver of all 19 patients in group A with pre-LT TAE showed tumor necrosis. Significant tumor necrosis from >85% to 100% was observed in 12 of the 19 patients (63.1%) after TAE. In the other 7 cases, <85% of tumor necrosis was found. The estimated median percentage of tumor necrosis was well correlated with the post-TAE CT finding and pathological specimen. Microscopic tumor invasion to the portal vein was found in 2 cases that were underestimated by the pre-operative imaging studies. Pathological evaluation of the explanted liver shows no discrepancy between the clinical staging and pathological finding. Downgrading of HCC was achieved in all 7 patients in group A2 to meet the Milan criteria (Figure 1).
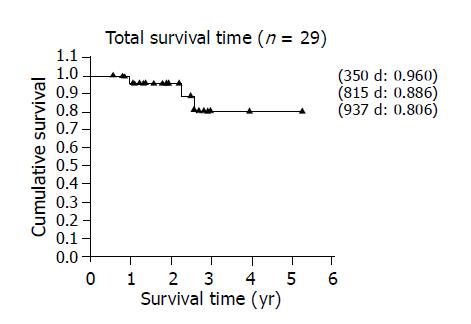
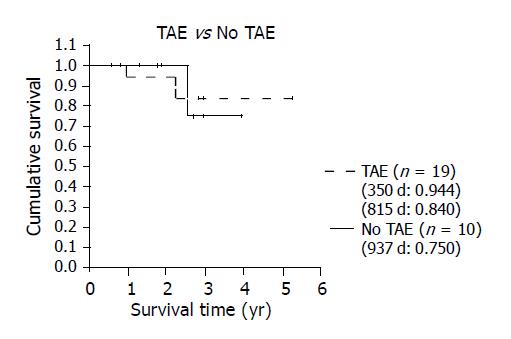
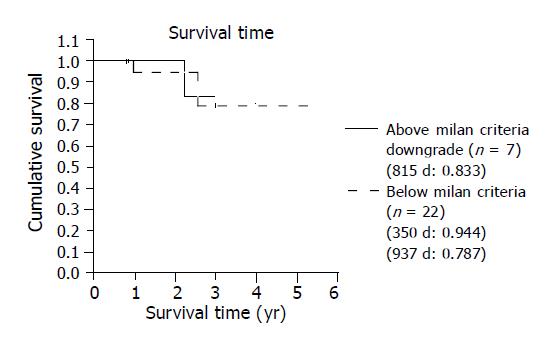
After LT, all 29 patients were followed for 747.83±391.66 d (mean±SD, range: 204-1920) in the outpatient clinic with ultrasound, CT, and liver function tests. The overall 5-year actuarial survival rate was 80.6% (Figure 2). The survival rates were different between group A (i.e., TAE group) and group B (i.e., non-TAE group) with the former showing a better 5-year survival (84%) than the 4-year survival in the latter (75%) (Figure 3). The 3-year survival of the 7 patients who exceeded the Milan criteria pre-LT and were downgraded by TAE (group A2) was 83% which is better than the patients that met the criteria pre-LT (n = 22) (Figure 4). In group A1, one patient suffered from lung metastasis 6 mo after LT and died one year later. Microscopic tumor invasion to the portal vein was also noted in the explanted liver of that patient. The other mortality occurred 2.2 years after LT in the downgraded group (group A2). However, the mortality was due to primary lung cancer unrelated to recurrent HCC. In the non-TAE group (group B), one patient was lost due to the recurrence of hepatitis C.
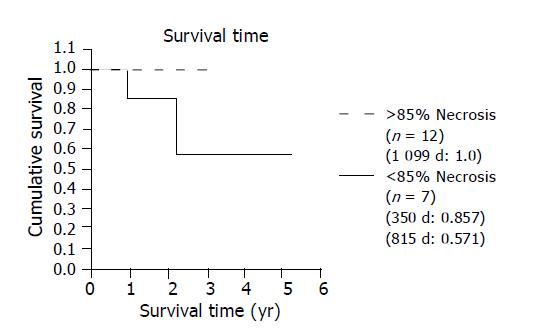
Among the 12 patients, whose tumors had undergone necrosis >85%, no recurrent tumor was found and their disease-free survival (100% at 3 years) was significantly better than the others who showed <85% tumor necrosis (57.1% at 3 years) (Figure 5) or who did not have TAE (75% at 3 years).
Although the outcome of LT has proved encouraging in the treatment of advanced HCC, the shortage of organs dissuades the policy for organ allocation for malignant disease in Taiwan. The number and size of tumors are considered major factors associated with the risk of tumor recurrence and survival[4]. For patients with liver tumor size and number exceeding the Milan criteria for LT, TAE was beneficial in controlling tumor growth, effectively decreasing tumor size, and allowing a wider range of patients to undergo liver surgery and achieve better survival. Downgrading or total necrosis of the tumor induced by TAE was also associated with improved disease-free survival after resection. In this study, we investigated the influence of TAE on patients undergoing LT for HCC associated with liver cirrhosis. Although the overall 5-year survival in LT for HCC is 80.6%, which is far behind the overall patients’ survival (after undergoing LT in our program) of 95% at 5 years, preoperative TAE followed by LT is associated with a better outcome and may be a sensible therapeutic strategy for selected patients with HCC. In our study, TAE before LT appears to be most useful in patients who exceed selection criteria of a single lesion smaller than 5 cm or three lesions smaller than 3 cm. Response to TAE in the form of downgrading or necrosis >85% of the tumor was observed in 63% (12/19) of patients and associated with increased disease-free survival. Response to TAE in these patients has important clinical implications as patients with large tumors are generally considered poor candidates for LT, especially when presenting with multinodular disease. Our results showed that downgrading by TAE is associated with low incidence of recurrence after LT comparable to that in patients with smaller tumors and should be regarded as a strong argument for patients with advanced HCC to proceed to LT.
The significance of the role of TAE in pre-LT treatment was further underscored by the fact that although group A patients had more advanced HCC and were significantly older compared to those in group B (P<0.05, Table 1), the former actually enjoyed a better survival rate than the latter after transplant.
Cadaveric LT is an excellent treatment for early HCC. Its use, however, is limited by the shortage of grafts. As a result of prolonged waiting period before transplantation, tumor progression may counteract the benefit of LT. An estimated 30% of patients develop contra-indications to the procedure while waiting for a suitable donor and up to 10% of patients with HCC on transplant waiting list die before undergoing LT[8,9]. Surgical resection of the tumor is an optimal bridging treatment, which has been anecdotally proposed in many centers[10]. However, acceptable liver function is the prerequisite for hepatectomy or tumor resection. In fact, less than 30% of patients who have advanced liver cirrhosis would tolerate liver resection[1,2]. So TAE is another treatment of choice in these cirrhotic patients to halt or delay tumor progression and to reduce the impact of a long waiting list and donor shortage. Presence of vascular invasion, number of satellite nodules, natural history of tumor behavior and response to TAE are powerful predictors of survival in patients with HCC. Angiography and TAE can demonstrate and offer that additional information. Patients with poor prognostic criteria may be removed from the waiting list.
From the experience using animal model, the nature of the injected material and the rate of injection had a significant impact on the actual amount of embolizer that reaches the tumor, the adjacent parenchyma, and the portal vein. The pharmacokinetics is especially important for those liquid materials that are not soluble in blood, such as lipiodol/ethanol mixture, to pass from the hepatic artery and to the portal vein through the presinusoidal communication to create a dual artery and portal vein embolization[11,12]. On the kinetics of the flow, slow injection can produce small droplets of the liquid embolizer that are carried along with the high velocity main stream towards the feeding vessels of the tumor. When the velocity of the main blood flow slows down during embolization, the embolizer will be evenly distributed inside the tumor and also the adjacent liver parenchyma according to the velocity of the blood vessels. Our results suggest that preoperative TAE can achieve better results than those cases with similar tumor sizes but received LT without prior TAE. It indicates that the therapeutic effect of the transhepatic artery approach by using lipiodol/ethanol mixture is an effective modality in the treatment of HCC especially when combined with LT.
Significant tumor necrosis is an important factor that contributed to the excellent outcome after TAE in our study. Our data revealed that recurrence was infrequent in those patients with TAE-induced extensive tumor necrosis who showed an excellent 100% disease-free survival at 3 years. It is superior to the incomplete embolization group with less than 85% tumor necrosis (57.1% at 3 years) or who did not have TAE (75% at 3 years) before LT. Almost all of our patients showed a marked response to pre-transplant TAE, 63% (12/19) of the patients had >85% tumor necrosis or at least greater than 50% tumor size reduction in the explanted livers. This high response rate can possibly be explained by the superselective embolization, slow injection of the embolizer, dual hepatic artery and portal vein embolization, and the strategy of repeated TAE sessions within a short period of time to achieve maximal necrosis. The procedure was well- tolerated in the majority of patients and caused almost no significant complications.
Hepatic artery injury during TAE is considered a risk factor for LT that may impair post-transplant survival especially in the living donor liver transplant. Delicate interventional technique, highly specific selection of intrahepatic artery by using microcatheter, and slow injection of embolizer using microinfusion pump to prevent reflux of the agents can minimize injury to the major hepatic artery. From our experience, TAE prior to LT did not increase surgical difficulty in hepatic artery dissection and anastomosis. No graft loss due to hepatic artery injury was observed in our 19 TAE patients.
In addition to the tumor size and number, vascular invasion is another important predictor of outcome after transplant. Early lung metastasis was also noted in one of our cases with microscopic tumor invasion to the portal vein. Although TAE was performed by slowly infusing the mixture of lipiodol and ethanol into the artery supplying the tumor until dual hepatic artery and portal vein embolization, early distant metastasis still cannot be prevented. Unfortunately, the diagnosis of microscopic vascular invasion can only be made under microscope in vitro and cannot be predicted or detected by any laboratory tests, imaging modalities, and even invasive procedures such as biopsy and angiography. Since advanced HCC (stage 4) may still achieve 20% 5-year survival post-LT in comparison with 100% mortality without operation[13], all HCC patients without extrahepatic spread should be offered LT. The major limiting factors have been organ shortage and cost. On the basis of the probability of early recurrence, candidates with vascular invasion should be excluded from the transplantation waiting list.
Pathologic analysis showed that the percentage of tumor necrosis correlated with the results of post-TAE CT. Besides, post-TAE CT, with lipiodol stasis in HCC, can show nodules previously ignored by CT, ultrasound, and angiography, contributing to a more accurate staging of the disease[14,15]. It indicated that post-TAE CT is a good examination modality that can be used in the pre-transplant survey that includes patient selection and outcome prediction after LT. Precise assessment of the size, number, and percentage of tumor necrosis after TAE are among the most powerful predictors of survival in patients with HCC. In addition to these factors, natural history of tumor behavior can be incorporated into future treatment planning. Uncontrolled tumor growth after TAE that does not meet the criteria and macroscopic vascular invasion may not be good candidates for transplantation and could therefore be removed from the waiting list. Other patients with insufficient tumor necrosis after TAE but within the criteria may be selected for early transplantation.
In conclusion, our results show a low risk of recurrent HCC in patients treated with preoperative TAE before LT. These results also provide evidence to redefine the current rationale behind organ allocation for malignant liver diseases. The combination of the improved survival rate noted in this study and the development of living donor LT may potentially revolutionize the current scoring system and scheme of organ allocation that would advocate organ allocation for patients with advanced HCC. For those patients, Pre-LT TAE may be considered the therapeutic strategy of choice that may reduce their dropout rate for LT to achieve better patient survival and quality of life.
Edited by Li WZ Language Editor Elsevier HK
| 1. | Lin TM, Chen CJ, Tsai SF, Tsai TH. Hepatoma in Taiwan. J Natl Public Health Asso. 1988;91-100. |
| 2. | Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 3. | Gondolesi GE, Roayaie S, Muñoz L, Kim-Schluger L, Schiano T, Fishbein TM, Emre S, Miller CM, Schwartz ME. Adult living donor liver transplantation for patients with hepatocellular carcinoma: extending UNOS priority criteria. Ann Surg. 2004;239:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 162] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 4. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5312] [Article Influence: 183.2] [Reference Citation Analysis (0)] |
| 5. | Lin DY, Lin SM, Liaw YF. Non-surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 1997;12:S319-S328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Kan Z, Wallace S. Transcatheter liver lobar ablation: an experimental trial in an animal model. Eur Radiol. 1997;7:1071-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Cheng Y, Kan Z, Chen C, Huang T, Chen T, Yang B, Ko S, Lee T. Efficacy and safety of preoperative lobar or segmental ablation via transarterial administration of ethiodol and ethanol mixture for treatment of hepatocellular carcinoma: clinical study. World J Surg. 2000;24:844-850; discussion 850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Sarasin FP, Majno PE, Llovet JM, Bruix J, Mentha G, Hadengue A. Living donor liver transplantation for early hepatocellular carcinoma: A life-expectancy and cost-effectiveness perspective. Hepatology. 2001;33:1073-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 195] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 9. | Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1271] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 10. | Belghiti J, Cortes A, Abdalla EK, Régimbeau JM, Prakash K, Durand F, Sommacale D, Dondero F, Lesurtel M, Sauvanet A. Resection prior to liver transplantation for hepatocellular carcinoma. Ann Surg. 2003;238:885-892; discussion 892-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 319] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 11. | Kan Z, Wallace S. Sinusoidal embolization: impact of iodized oil on hepatic microcirculation. J Vasc Interv Radiol. 1994;5:881-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Kan Z, Ivancev K, Lunderquist A. Peribiliary plexa--important pathways for shunting of iodized oil and silicon rubber solution from the hepatic artery to the portal vein. An experimental study in rats. Invest Radiol. 1994;29:671-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Penn I. Hepatic transplantation for primary and metastatic cancers of the liver. Surgery. 1991;110:726-734; discussion 734-735. [PubMed] |
| 14. | Ohishi H, Uchida H, Ohue S, Yoshimura H, Yoshioka T, Matsuo N, Yoshida H, Fukai Y. Computed tomography detection of small daughter nodules in hepatocellular carcinoma after iodized oil infusion into the hepatic artery. J Comput Tomogr. 1988;12:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Novell R, Dusheiko G, Hilson A, Dick R, Begent R, Hobbs K. Lipiodol computed tomography for small hepatocellular carcinomas. Lancet. 1991;337:729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |