Published online Jan 7, 2005. doi: 10.3748/wjg.v11.i1.51
Revised: December 18, 2003
Accepted: January 12, 2004
Published online: January 7, 2005
AIM: To investigate the mutations of the 5’ noncoding region of BCL-6 gene in Chinese patients with primary gastric lymphomas.
METHODS: PCR and direct DNA sequencing were used to identify BCL-6 gene mutations in the 5’ noncoding region in 29 cases of gastric diffuse large B-cell lymphoma (DLBCL) and 18 cases of gastric mucosa-associated lymphoid tissue (MALT) lymphoma as well as 10 cases of reactive hyperplasia of lymph node (LRH).
RESULTS: Six of 29 gastric DLBCLs (20.7%), 4 of 18 gastric MALT lymphomas (22.2%) and 1 of 10 LRHs(10%) were found to have mutations. All mutations were single-base substitutions and the frequency of single-base changes was 0.20×10-2 -1.02×10-2 per bp.
CONCLUSION: Point mutations in the 5’ noncoding region of BCL-6 gene are found in Chinese patients with primary gastric DLBCLs and MALT lymphomas, suggesting that they may, in some extent, participate in the pathogenesis of primary gastric DLBCLs and MALT lymphomas.
-
Citation: Min DL, Zhou XY, Yang WT, Lu HF, Zhang TM, Zhen AH, Cao PZ, Shi DR. Point mutation of 5’ noncoding region of
BCL-6 gene in primary gastric lymphomas. World J Gastroenterol 2005; 11(1): 51-55 - URL: https://www.wjgnet.com/1007-9327/full/v11/i1/51.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i1.51
BCL-6 protooncogene, which is located at chromosome 3q27 encoding a POZ/zinc finger sequence-specific transcription repressor, is one of the three genes most commonly implicated in non Hodgkin’s lymphoma (the other two are BCL-2 and c-myc genes)[1-7]. Clonal BCL-6 gene rearrangements are observed in 30% to 40% of nodal DLBCLs and 5% to 10% of nodal follicular lymphomas (FLs)[3,4]. These rearrangements are clustered within a highly conserved 4.0-kb regulatory region spanning the promoter, resulting in BCL-6 expression deregulation by a heterologous promoter from the partner chromosomes[8-10]. It is believed that the deregulation of BCL-6 gene expression contributes to lymphomagenesis. Recent studies[11-14] also indicate that BCL-6 gene may be alterd by somatic mutations clustered within the 5’ noncoding regions of this gene. These mutations have been found in cases displaying either normal or rearranged BCL-6 alleles, indicating their independence of chromosomal translocations. The sequences affected by these mutations are adjacent to the BCL-6 promotor region and overlapped with MBR. The mutation frequency is more than 70% in nodal DLBCL, which is much higher than that of rearrangement, and the high frequency, tumor specificity and location in the proximity of BCL-6 regulatory regions of these mutations suggest that these genetic alterations may play a role in lymphomagenesis[15-21]. However, most of BCL-6 mutations are focused on lymphomas originated from lymph node; lymphomas originated from extranodal site were less investigated. This study was aimed to investigate mutations of the 5’ noncoding region of BCL-6 gene in Chinese patients with primary gastric lymphomas.
A total of 47 cases of paraffin-embedded primary gastric lymphomas, including 29 cases of DLBCL and 18 cases of MALT lymphoma were collected from the Department of Pathology, Cancer Hospital of Fudan University. In addition, 10 paraffin-embedded LRH specimens were included for control. Mean patient age was 56 years, male/female ratio was 1.2:1. In all instances, specimens were collected at diagnosis before specific therapy. Diagnosis was based on histopathological and immunophenotypic analysis of cell surface markers and immunogenotypic analysis of antigen receptor gene rearrangements. All lymphoma specimens were classified according to the new World Health Organization (WHO) classification of lymphoid neoplasms proposed in 1997[22]. The samples which were diagnosed before the advent of the new WHO lymphoma classification were reinvestigated after proper immunohistochemical studies to meet the criteria of the new classification.
Six μm thick sections from paraffin blocks were dewaxed in xylene, rinsed in ethanol, stained with hematoxylin and air-dried. The desired tumor areas were obtained by microdissection using scalpels under an upside-down light microscope. In most cases, the fraction of malignant cells was ≥85%. Genomic DNA was extracted from collected cells, which were subjected to lysis in 0.5-1.0 mL cell lysis buffer containing 100 mmol/L Tris-Cl pH8.5, 20 mmol/L EDTA, 20 mmol/L NaCl and 2.0% SDS, 0.5-2.0 mg/mL proteinase K and then to conventional phenol/chloroform extraction and ethanol precipitation.
Two PCR products encompassing fragments E1.11 and E1.12 and spanning 490 bp were amplified by primer 5’-AGGAAGGAGGGGAATTAG-3’ (sense), 5’-AAGCAGTTTGCAAGCGAG-3’ (antisense) (for E1.11) and primer 5’-TTCTCGCTTGCAAACTGC-3’ (sense), 5’-CACGATACTTCATCTCATC-3’ (antisense) (for E1.12) respectively. The choice of these fragments was based on the fact that >95% of BCL-6 mutations detected in DLBCL were within these regions[11]. The first nucleotide of the amplified BCL-6 gene region corresponding to the first nucleotide of the sense primer of E1.11 fragment was arbitrarily defined as position +1 (GenBank accession number AF191831). PCR was performed in a final volume of 25 μL containing 10 pmoL of each primer, 10 mmol/L Tris-Cl (pH8.5), 50 mmol/L KCl, 1.5 mmol/L MgCl2, 200 μmol/L of each dNTP, 1.5 units of Taq polymerase (Promega,USA) and 1000 ng of genomic DNA. The PCR conditions were as follows: denaturation at 94 °C for 5 min, 35 cycles were performed, each consisting of denaturation at 94 °C for 1 min, annealing at 57 °C (for E1.11) or at 52 °C (for E1.12) for 30 s, extension at 72 °C for 45 s, followed by a final extension at 72 °C for 7 min. PCR was performed in a Perkin Elmer 9 700 GeneAmp PCR system. For each PCR, a control with no added template was used to check for contamination. The amplified fragments were checked using 2% agarose gel electrophoresis.
PCR product of 20 μL was purified with a QIA quick spin column according to the manufacturer’s instructions. The purified DNA fragments were directly sequenced on an ABI PRISM 310 DNA sequencer, using ABI PRISM big dye terminator kit as recommended by the manufacturer. Both the sense and antisense strands were sequenced and each fragment with suspected mutations was sequenced at least twice. Controls were also sequenced to ensure the fidelity and reliability of the sequencing results. Sequence was aligned with the BCL-6 germline sequence (GenBank accession number AF191831) by the Internet blast programme (http://www.ncbi.nih.gov/blast). The frequency of mutation was calculated by the detected length of gene fragment (490 bp) divided by the number of mutations.
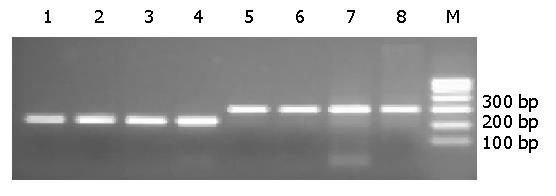
The amplified E1.11 and E1.12 fragments of BCL-6 gene were shown by 2% agarose gel electrophoresis (Figure 1).
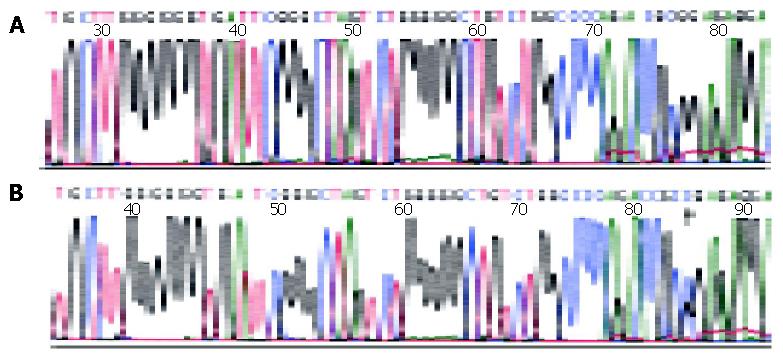
Six of 29 gastric DLBCLs (20.7%), 4 of 18 gastric MALT lymphomas (22.2%) and 1 of 10 LRHs (10%) were found to have mutations (Table 1). All mutations were single-base substitutions and the frequency of single-base changes in the 5’ noncoding region was as high as 0.20×10-2/bp to1.02×10-2/bp, similar to those of IgV gene hypermutation during antigen-stimulated clonal selection[23,24]. Single-base changes in the BCL-6 gene 5’ noncoding region and some of the DNA sequencing results from the positive cases are shown in Table 2 and Figure 2.
| Sample | Mutated cases (%) |
| DLBCL | 6/29 (20.7) |
| MALTL | 4/18 (22.2) |
| LRH | 1/10 (10.0) |
| Sample | Diagnosis | Substitution mutations |
| 1 | DLBCL | G→C (397), G→A (403) |
| 2 | DLBCL | G→C (397), C→T (419) |
| 3 | DLBCL | C→A (624) |
| 4 | DLBCL | T→A (346), G→A (391), G→A (402),G→A (694), G→A (669) |
| 5 | DLBCL | G→C (397) |
| 6 | DLBCL | G→A (322), T→C (444) |
| 7 | MALTL | G→T (321), G→C (397) |
| 8 | MALTL | C→A (665) |
| 9 | MALTL | G→C (397), C→G (419) |
| 10 | MALTL | C→T(123), G→C (397), T→C (484) |
| 11 | LRH | G→C (397) |
The commonest site of extranodal lymphomas is located in the gastrointestinal (GI) tract, particularly in the stomach[25-27]. It is uncertain whether primary lymphoma of the stomach is pathogenetically different from that of its nodal counterpart. This study was to analyze mutations in the 5’ noncoding region of BCL-6 gene in Chinese patients with primary gastric lymphoma.
BCL-6 gene was originally identified by virtue of its involvement in chromosomal translocations affecting band 3q27 in NHL[1,2]. BCL-6 gene contains 10 exons and encodes for a 3.8-kb mRNA that is translated into a 706-amino acid nuclear phosphoprotein characterized by six Kruppel-type C-terminal zinc-finger motifs that have been shown to recognize specific DNA sequences. BCL-6 protein has been identified as a potent transcriptional repressor of promoters linked to its DNA target sequences, and is down-regulated during B cell differentiation to plasma cells. BCL-6 gene has been shown to be a multifunctional gene, regulating important genes involved in B-cell differentiation (blimp-1, IP-10, and others) and cell-cycle control (such as c-myc, p27KIP1, and cyclinD2)[20,28-36].
The 5’ noncoding region of BCL-6 gene contains regulatory elements for its expression. Ohashi et al[37] found that the 1.5-kb promoter region of BCL-6 was characterized by a TATA box and a number of potential regulatory elements such as CACCC, E-box and GATA-1 sites, which may be responsible for the low expression of the gene in normal lymphoid tissues and non-germinal-center derived lymphoid malignancies. Previous studies[11-15] suggest that BCL-6 mutations might have functional significance, based on their frequency and clustering in the proximity of the BCL-6 promoter. This is supported by in vitro studies showing that mutations might alter the transcriptional activity of BCL-6. In most tumor cases, mutations are somatic, multiple, often biallelic. And clusteres in the 5’ regulatory sequences at frequencies of 7×10-4 to 1.6×10-2/bp are comparable with those of IgV genes in B cells[23]. Hypermutations of the 5’ noncoding region in BCL-6 gene may cause disordered regulatory cascades of gene expression, thus leading to the destruction of germinal centers and the maintenance of immature B cell status, which could play a key role in the development of germinal center-derived DLBCL[38-40]. Gaidano et al[12] demonstrated that one single mutation of BCL-6 gene 5’ regulatory region was able to alter its transcriptional activity, suggesting a pivotal role in the tumorigenesis of germinal center-derived lymphomas[33-39]. In vitro studies aimed at transfecting normal B cells with mutated BCL-6 alleles can clarify the precise pathogenetic implications of these mutations.
BCL-6 mutations represent a marker of germinal center (GC) or post-GC cells because in normal lymphoid tissues, they occur in approximately 30 -50% of GC and memory B cells, whereas they are absent in pre-GC and virgin B cells[12-16]. Thus, BCL-6 mutations are proposed as a genetic marker for defining the histogenesis of B-cell lymphoproliferations, and accumulation of BCL-6 mutations might result from ectopic activity of the IgV gene hypermutation mechanism involving sequences displaying no homology with antigen receptor loci[23,24].
It has been reported that the 5’ non-coding region point mutation of BCL-6 gene occur in 73% nodal DLBCLs and 45% FLs in Western populations[11]. But in this study, we found that the mutational incidence of the 5’ noncoding region of BCL-6 gene was 20.7% in Chinese patients with primary gastric DLBCLs which is significantly lower than that in Western populations. This result is in accordance with our previous reports[41] in nodal DLBCL which showed that the mutational incidence of the 5’ noncoding region of BCL-6 gene was 18.4%. The differences of mutational incidence between our study and Western reports in DLBCL might be related to the differences in the screened regions, the distinct aspects of races and social-economic environments and even the different molecular pathologenesis of DLBCL[42].
Gastric MALT lymphoma is of B-cell origin and has a very strong association with Helicobacter pylori infection[43-45]. It has been found that eradication of the infection with antibiotics may lead to regression of gastric MALT lymphoma. In gastric MALT lymphoma, the results of investigations on BCL-6 mutations are variable; BCL-6 mutations were found in 2 out of 4 cases in Liang’s study[26], but mutations were absent in all 16 cases tested in Gaidano’s study[27]. In this study, mutations of the 5’ noncoding region of BCL-6 gene were detected in 4 of 18 gastric MALT lymphomas (22.2%). Because MALT lymphoma has been traditionally viewed as proliferation of marginal region cells, the occurrence of BCL-6 mutations in a fraction of MALT lymphomas suggests that the histogenesis of MALT lymphoma might be more heterogenerous than previously thought[46-50]. This is consistent with the hypothesis that the fraction of MALT lymphoma with BCL-6 somatic mutation might be derived from germinal center-related B cells. Kwon et al[42] investigated that tissues obtained from the marginal zone of Peyer’s patch by microdissection revealed no BCL-6 mutations by PCR-SSCP analysis, whereas tissues from gastric MALT lymphomas were shown to have BCL-6 mutations in 11 of 13 (86.4%). They believe that the acquisition process of BCL-6 mutations by marginal zone cells might be involved in the lymphomagenesis of gastric MALT lymphoma.
Lossos et al[19] demonstrated that mutations occurred in the 5’ regulatory region of BCL-6 gene were ongoing. It is possible that as a result of ongoing BCL-6 gene somatic mutations, lymphoma cells become heterogeneous, and a mutational variant having a selective growth advantage because of BCL-6 overexpression gives rise to the higher-grade of NHL lymphoma cells.
In the present study, seven recurrent mutations (G→C) were identified at position 397, suggesting that this position may be a mutational hot spot. Several other studies[13,20,41] also reported mutations at this position, but Lossos et al[13] thought that the mutation at position 397 might be a polymorphism, because it was also observed in two samples of T cells from patients. Further studies are needed to determine whether the position 397 (G→C) is a real mutation or just a polymorphism.
In conclusion, point mutations of the 5’ noncoding region of BCL-6 gene suggest that they may, in some extent, participate in the pathogenesis of primary gastric DLBCLs and MALT lymphomas.
We thank Professor Xiong-Zeng Zhu (Department of Pathology, Cancer Hospital of Fudan University, Shanghai, China) for his review of the manuscript and helpful suggestions.
Edited by Wang XL Proofread by Chen WW
| 1. | Ye BH, Rao PH, Chaganti RS, Dalla-Favera R. Cloning of bcl-6, the locus involved in chromosome translocations affecting band 3q27 in B-cell lymphoma. Cancer Res. 1993;53:2732-2735. [PubMed] |
| 2. | Ye BH, Lista F, Lo Coco F, Knowles DM, Offit K, Chaganti RS, Dalla-Favera R. Alterations of a zinc finger-encoding gene, BCL-6, in diffuse large-cell lymphoma. Science. 1993;262:747-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 532] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 3. | Lo Coco F, Ye BH, Lista F, Corradini P, Offit K, Knowles DM, Chaganti RS, Dalla-Favera R. Rearrangements of the BCL6 gene in diffuse large cell non-Hodgkin's lymphoma. Blood. 1994;83:1757-1759. [PubMed] |
| 4. | Bastard C, Deweindt C, Kerckaert JP, Lenormand B, Rossi A, Pezzella F, Fruchart C, Duval C, Monconduit M, Tilly H. LAZ3 rearrangements in non-Hodgkin's lymphoma: correlation with histology, immunophenotype, karyotype, and clinical outcome in 217 patients. Blood. 1994;83:2423-2427. [PubMed] |
| 5. | Bernardin F, Collyn-d'Hooghe M, Quief S, Bastard C, Leprince D, Kerckaert JP. Small deletions occur in highly conserved regions of the LAZ3/BCL6 major translocation cluster in one case of non-Hodgkin's lymphoma without 3q27 translocation. Oncogene. 1997;14:849-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Ye BH. Proto-oncogene BCL-6 and the pathogenesis of non-Hodgkin’s lymphoma. Einstein quart. J Biol Med. 1999;16:130-143. |
| 7. | Kramer MH, Hermans J, Wijburg E, Philippo K, Geelen E, van Krieken JH, de Jong D, Maartense E, Schuuring E, Kluin PM. Clinical relevance of BCL2, BCL6, and MYC rearrangements in diffuse large B-cell lymphoma. Blood. 1998;92:3152-3162. [PubMed] |
| 8. | Chen W, Butler M, Rao PH, Chaganti SR, Louie DC, Dalla-Favera R, Chaganti RS. The t(2; 3)(q21; q27) translocation in non-Hodgkin's lymphoma displays BCL6 mutations in the 5' regulatory region and chromosomal breakpoints distant from the gene. Oncogene. 1998;17:1717-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Tamura A, Miura I, Iida S, Yokota S, Horiike S, Nishida K, Fujii H, Nakamura S, Seto M, Ueda R. Interphase detection of immunoglobulin heavy chain gene translocations with specific oncogene loci in 173 patients with B-cell lymphoma. Cancer Genet Cytogenet. 2001;129:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Sanchez-Izquierdo D, Siebert R, Harder L, Marugan I, Gozzetti A, Price HP, Gesk S, Hernandez-Rivas JM, Benet I, Solé F. Detection of translocations affecting the BCL6 locus in B cell non-Hodgkin's lymphoma by interphase fluorescence in situ hybridization. Leukemia. 2001;15:1475-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Migliazza A, Martinotti S, Chen W, Fusco C, Ye BH, Knowles DM, Offit K, Chaganti RS, Dalla-Favera R. Frequent somatic hypermutation of the 5' noncoding region of the BCL6 gene in B-cell lymphoma. Proc Natl Acad Sci USA. 1995;92:12520-12524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 276] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Gaidano G, Carbone A, Pastore C, Capello D, Migliazza A, Gloghini A, Roncella S, Ferrarini M, Saglio G, Dalla-Favera R. Frequent mutation of the 5' noncoding region of the BCL-6 gene in acquired immunodeficiency syndrome-related non-Hodgkin's lymphomas. Blood. 1997;89:3755-3762. [PubMed] |
| 13. | Lossos IS, Levy R. Mutation analysis of the 5' noncoding regulatory region of the BCL-6 gene in non-Hodgkin lymphoma: evidence for recurrent mutations and intraclonal heterogeneity. Blood. 2000;95:1400-1405. [PubMed] |
| 14. | Capello D, Vitolo U, Pasqualucci L, Quattrone S, Migliaretti G, Fassone L, Ariatti C, Vivenza D, Gloghini A, Pastore C. Distribution and pattern of BCL-6 mutations throughout the spectrum of B-cell neoplasia. Blood. 2000;95:651-659. [PubMed] |
| 15. | Vitolo U, Botto B, Capello D, Vivenza D, Zagonel V, Gloghini A, Novero D, Parvis G, Calvi R, Ariatti C. Point mutations of the BCL-6 gene: clinical and prognostic correlation in B-diffuse large cell lymphoma. Leukemia. 2002;16:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Mascle X, Albagli O, Lemercier C. Point mutations in BCL6 DNA-binding domain reveal distinct roles for the six zinc fingers. Biochem Biophys Res Commun. 2003;300:391-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Yavuz AS, Monson NL, Yavuz S, Grammer AC, Longo N, Girschick HJ, Lipsky PE. Different patterns of bcl-6 and p53 gene mutations in tonsillar B cells indicate separate mutational mechanisms. Mol Immunol. 2002;39:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Chen PM, Yang MH, Yu IT, Lin JT, Lin YC, Fan FS, Wang WS, Yen CC, Chiou TJ, Liu JH. Low incidence of BCL-6 gene alterations for diffuse large B-cell lymphomas in Taiwan Chinese. Cancer. 2002;94:2635-2644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Lossos IS, Levy R. Higher-grade transformation of follicle center lymphoma is associated with somatic mutation of the 5' noncoding regulatory region of the BCL-6 gene. Blood. 2000;96:635-639. [PubMed] |
| 20. | Artiga MJ, Sáez AI, Romero C, Sánchez-Beato M, Mateo MS, Navas C, Mollejo M, Piris MA. A short mutational hot spot in the first intron of BCL-6 is associated with increased BCL-6 expression and with longer overall survival in large B-cell lymphomas. Am J Pathol. 2002;160:1371-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Capello D, Carbone A, Pastore C, Gloghini A, Saglio G, Gaidano G. Point mutations of the BCL-6 gene in Burkitt's lymphoma. Br J Haematol. 1997;99:168-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD. The World Health Organization classification of neoplastic diseases of the haematopoietic and lymphoid tissues: Report of the Clinical Advisory Committee Meeting, Airlie House, Virginia, November 1997. Histopathology. 2000;36:69-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 291] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 23. | Pasqualucci L, Migliazza A, Fracchiolla N, William C, Neri A, Baldini L, Chaganti RS, Klein U, Küppers R, Rajewsky K. BCL-6 mutations in normal germinal center B cells: evidence of somatic hypermutation acting outside Ig loci. Proc Natl Acad Sci USA. 1998;95:11816-11821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 380] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 24. | Peng HZ, Du MQ, Koulis A, Aiello A, Dogan A, Pan LX, Isaacson PG. Nonimmunoglobulin gene hypermutation in germinal center B cells. Blood. 1999;93:2167-2172. [PubMed] |
| 25. | Barth TF, Döhner H, Möller P, Bentz M. Chromosomal aberrations in lymphomas of the gastrointestinal tract. Leuk Lymphoma. 1999;36:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Liang R, Chan WP, Kwong YL, Chan AC, Xu WS, Au WY, Srivastava G, Ho FC. Bcl-6 gene hypermutations in diffuse large B-cell lymphoma of primary gastric origin. Br J Haematol. 1997;99:668-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Gaidano G, Capello D, Gloghini A, Fassone L, Vivenza D, Ariatti C, Migliazza A, Saglio G, Carbone A. Frequent mutation of bcl-6 proto-oncogene in high grade, but not low grade, MALT lymphomas of the gastrointestinal tract. Haematologica. 1999;84:582-588. [PubMed] |
| 28. | Falini B, Fizzotti M, Pileri S, Liso A, Pasqualucci L, Flenghi L. Bcl-6 protein expression in normal and neoplastic lymphoid tissues. Ann Oncol. 1997;8 Suppl 2:101-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Lossos IS, Jones CD, Warnke R, Natkunam Y, Kaizer H, Zehnder JL, Tibshirani R, Levy R. Expression of a single gene, BCL-6, strongly predicts survival in patients with diffuse large B-cell lymphoma. Blood. 2001;98:945-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 202] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 30. | Shaffer AL, Yu X, He Y, Boldrick J, Chan EP, Staudt LM. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000;13:199-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 660] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 31. | Dent AL, Vasanwala FH, Toney LM. Regulation of gene expression by the proto-oncogene BCL-6. Crit Rev Oncol Hematol. 2002;41:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | de Leval L, Ferry JA, Falini B, Shipp M, Harris NL. Expression of bcl-6 and CD10 in primary mediastinal large B-cell lymphoma: evidence for derivation from germinal center B cells? Am J Surg Pathol. 2001;25:1277-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Dogan A, Bagdi E, Munson P, Isaacson PG. CD10 and BCL-6 expression in paraffin sections of normal lymphoid tissue and B-cell lymphomas. Am J Surg Pathol. 2000;24:846-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 225] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 34. | Hori M, Qi CF, Torrey TA, Huppi K, Morse HC. The Bcl6 locus is not mutated in mouse B-cell lineage lymphomas. Leuk Res. 2002;26:739-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Stamatopoulos K, Kosmas C, Belessi C, Stavroyianni N, Kyriazopoulos P, Papadaki T. Molecular insights into the immunopathogenesis of follicular lymphoma. Immunol Today. 2000;21:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Sakashita C, Fukuda T, Okabe S, Kobayashi H, Hirosawa S, Tokuhisa T, Miyasaka N, Miura O, Miki T. Cloning and characterization of the human BAZF gene, a homologue of the BCL6 oncogene. Biochem Biophys Res Commun. 2002;291:567-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Ohashi K, Miki T, Hirosawa S, Aoki N. Characterization of the promoter region of human BCL-6 gene. Biochem Biophys Res Commun. 1995;214:461-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Hartatik T, Okada S, Okabe S, Arima M, Hatano M, Tokuhisa T. Binding of BAZF and Bc16 to STAT6-binding DNA sequences. Biochem Biophys Res Commun. 2001;284:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Gaidano G, Capello D, Cilia AM, Gloghini A, Perin T, Quattrone S, Migliazza A, Lo Coco F, Saglio G, Ascoli V. Genetic characterization of HHV-8/KSHV-positive primary effusion lymphoma reveals frequent mutations of BCL6: implications for disease pathogenesis and histogenesis. Genes Chromosomes Cancer. 1999;24:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Capello D, Gaidano G. Molecular pathophysiology of indolent lymphoma. Haematologica. 2000;85:195-201. [PubMed] |
| 41. | Zhou XY, Zhu WP, Zhang TM, Li XM, Jin AP, Sun MH, Zhu XZ. Mutation of 5'noncoding region of the bcl-6 gene in diffuse large B cell lymphomas. Zhonghua BingLiXue ZaZhi. 2003;32:10-13. [PubMed] |
| 42. | Kwon MS, Go JH, Choi JS, Lee SS, Ko YH, Rhee JC, Ree HJ. Critical evaluation of Bcl-6 protein expression in diffuse large B-cell lymphoma of the stomach and small intestine. Am J Surg Pathol. 2003;27:790-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Go JH, Yang WI, Ree HJ. Mutational analysis of the 5' noncoding region of the bcl-6 gene in primary gastric lymphomas. Mod Pathol. 2001;14:410-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 44. | Xue FB, Xu YY, Wan Y, Pan BR, Ren J, Fan DM. Association of H. pylori infection with gastric carcinoma: a Meta analysis. World J Gastroenterol. 2001;7:801-804. [PubMed] |
| 45. | Morgner A, Miehlke S, Stolte M, Neubauer A, Alpen B, Thiede C, Klann H, Hierlmeier FX, Ell C, Ehninger G. Development of early gastric cancer 4 and 5 years after complete remission of Helicobacter pylori associated gastric low grade marginal zone B cell lymphoma of MALT type. World J Gastroenterol. 2001;7:248-253. [PubMed] |
| 46. | Mateo MS, Mollejo M, Villuendas R, Algara P, Sanchez-Beato M, Martínez P, Piris MA. Molecular heterogeneity of splenic marginal zone lymphomas: analysis of mutations in the 5' non-coding region of the bcl-6 gene. Leukemia. 2001;15:628-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Maes M, Depardieu C, Dargent JL, Hermans M, Verhaeghe JL, Delabie J, Pittaluga S, Troufléau P, Verhest A, De Wolf-Peeters C. Primary low-grade B-cell lymphoma of MALT-type occurring in the liver: a study of two cases. J Hepatol. 1997;27:922-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Liang R, Chan WP, Kwong YL, Chan AC, Xu WS, Srivastava G. Mutation of the 5' noncoding region of the BCL-6 gene in low-grade gastric lymphoma of the mucosa-associated lymphoid tissue. Cancer Genet Cytogenet. 1998;102:110-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Starostik P, Greiner A, Schultz A, Zettl A, Peters K, Rosenwald A, Kolve M, Müller-Hermelink HK. Genetic aberrations common in gastric high-grade large B-cell lymphoma. Blood. 2000;95:1180-1187. [PubMed] |
| 50. | Yoshino T, Akagi T. Gastric low-grade mucosa-associated lymphoid tissue lymphomas: their histogenesis and high-grade transformation. Pathol Int. 1998;48:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |