Published online Jan 7, 2005. doi: 10.3748/wjg.v11.i1.41
Revised: April 28, 2004
Accepted: June 18, 2004
Published online: January 7, 2005
AIM: Mechanisms underlying the chemopreventive effects of cyclooxygenase (COX) inhibitors remain elusive. We have previously shown that celecoxib but not indomethacin could prevent carcinogen-induced gastric cancer development in Wistar rats. This chemopreventive effect appeared to be independent of COX-2 and prostaglandin (PG) E2 suppression since the lowest PGE2 was obtained in indomethacin group. This study compared the cell kinetic changes in stomachs of rats after treatment with celecoxib (5, 10, 20 mg/(kg·d)) or indomethacin (3 mg/(kg·d)) to gain more insights into the chemopreventive mechanism.
METHODS: The apoptosis and proliferation indexes in gastric tumor, adjacent non-cancer tissues and normal gastric tissues were determined. Apoptosis was quantified by apoptotic nuclei counting and TUNEL, whereas proliferation was determined by Ki67 immunostaining.
RESULTS: Treatment with either celecoxib or indomethacin inhibited gastric tumor proliferation by more than 65% (P<0.02). However, celecoxib caused a dose-dependent increase in apoptosis (P<0.05) which was not seen in indomethacin-treated tumors (P = 0.54). The highest apoptosis to proliferation ratio was seen in tumors treated with celecoxib at 10 mg/(kg·d). Treatment with this dose of celecoxib was associated with the lowest incidence of gastric cancer development.
CONCLUSION: Our findings suggest that the difference in chemopreventive effects of indomethacin and celecoxib in this animal model of gastric carcinogenesis is largely due to the differential cell kinetic changes, which does not correlate with the degree of COX-2 and PG suppression.
- Citation: Yu J, Tang BD, Leung WK, To KF, Bai AH, Zeng ZR, Ma PK, Go MY, Hu PJ, Sung JJ. Different cell kinetic changes in rat stomach cancer after treatment with celecoxib or indomethacin: Implications on chemoprevention. World J Gastroenterol 2005; 11(1): 41-45
- URL: https://www.wjgnet.com/1007-9327/full/v11/i1/41.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i1.41
Gastric cancer is the leading cause of cancer deaths in China. Interestingly, treatment with non-steroidal anti-inflammatory drugs (NSAIDs) including aspirin has been shown to reduce the risk of gastric cancer development in epidemiological studies[1-3]. However, the molecular mechanisms underlying the chemopreventive effect of NSAIDs remain poorly understood. There is accumulating evidence that NSAIDs exert their anti-neoplastic effect by inhibition of COX and prostaglandin[4,5]. The COX enzyme has two isoforms. COX-1 is constitutively expressed whereas COX-2 isoform is inducible. Notably, overexpression of COX-2 is frequently detected in human gastric cancer[6,7]. This expression is associated with uncontrolled cell proliferation and differentiation, inhibition of apoptosis, increase in angiogenesis, metastasis and evasion of immunological surveillance[8,9]. Accordingly, suppression of COX-2 appears to be the mechanism underlying the chemopreventive effect of NSAIDs. Treatment with specific COX-2 inhibitors suppresses the growth of gastric cancer xenografts in nude mice by inducing apoptosis and suppressing replication of the neoplastic cells[10].
However, recent reports suggest that the anti-neoplastic effects of NSAIDs might be independent of COX inhibition[11-13]. It has been found that agents that do not inhibit COX-2, such as sulindac sulfone, could also induce apoptosis in vitro and inhibit colorectal carcinogenesis in animal models[14]. Moreover, the use of low dose aspirin, which has virtually no COX-2 inhibitory effect, could reduce colorectal adenoma development in high risk individuals[15].
Recently, we have examined the chemopreventive effect of specific COX-2 inhibitors (celecoxib) and non-selective COX inhibitors (indomethacin) in a rat model of gastric carcinogenesis[16]. We showed that treatment with celecoxib, but not indomethacin, beginning shortly after carcinogen administration inhibited the growth and development of gastric tumors. Intriguingly, both COX-2 and prostaglandin E2 levels were lower in indomethacin-treated group than in celecoxib treated group, suggesting that the chemopreventive effect of celecoxib may not be mediated by inhibition of COX-2 activity or prostaglandin production alone. The present study was designed to clarify the cell kinetic changes in stomachs of rats after treatment with celecoxib or indomethacin in order to gain more insights into the pathogenetic mechanism underlying the chemopreventive effect of celecoxib.
The details of animal experimentation were reported previously[16]. Briefly, 4 week-old grade 2 male Wistar rats (weighing around 60 g) were used. The rats were fed with food and water ad libitum and maintained on hardwood bedding under a 12-h light/dark cycle. Animals were weighed weekly during the experiments.
Primary gastric adenocarcinomas were induced by oral administration of N-methyl-N’-nitro-N-nitrosoguanidine (MNNG) as described previously[17,18]. MNNG (Fluko, Germany) was prepared every other day with distilled water into a concentration of 100 μg/mL and was given to rats as drinking water. In addition, 1 mL of 10% sodium chloride was given weekly by oral gavage in the initial 6 wk to enhance gastric cancer development[18]. All experiments were approved by the Sun Yat-Sen University Animal Experimentation and Ethics Committee.
Rats were randomly allocated to 6 different treatment groups as shown in Table 1: Group A: untreated control (n = 5), group B: MNNG control (n = 16), group C: MNNG plus celecoxib at 5 mg/(kg·d) (n = 17), group D: MNNG plus celecoxib at 10 mg/(kg·d) (n = 16), group E: MNNG plus celecoxib at 20 mg/(kg·d) (n = 16) and group F: MNNG plus indomethacin at 3 mg/(kg·d) (n = 16). The dosages of these drugs were based on corresponding human doses and previous animal chemopreventive studies[10,11]. All drug treatments were commenced on d 7 after the introduction of MNNG and continued for 40 wk. All animals were then sacrificed at the end of study.
| Group | Treatment | Total No. of rats | No. of rats with tumor | Tumor incidence (%) |
| A | Control | 5 | 0 | 0 |
| B | MNNG alone | 16 | 12 | 75.01 |
| C | MNNG+celecoxib 5 mg/kg | 17 | 12 | 70.6 |
| D | MNNG+celecoxib 10 mg/kg | 16 | 3 | 18.8 |
| E | MNNG+celecoxib 20 mg/kg | 16 | 5 | 31.3 |
| F | MNNG+indomethacin 3 mg/kg | 16 | 11 | 68.8 |
Gastric tumor (T), adjacent non-tumor site (NT), macroscopically normal gastric mucosa from non-tumor rats (N) in the same treatment group were obtained. In untreated control rats, normal gastric tissues were obtained as control (C). All gastric tissues were fixed in 10% buffered formalin for histological processing.
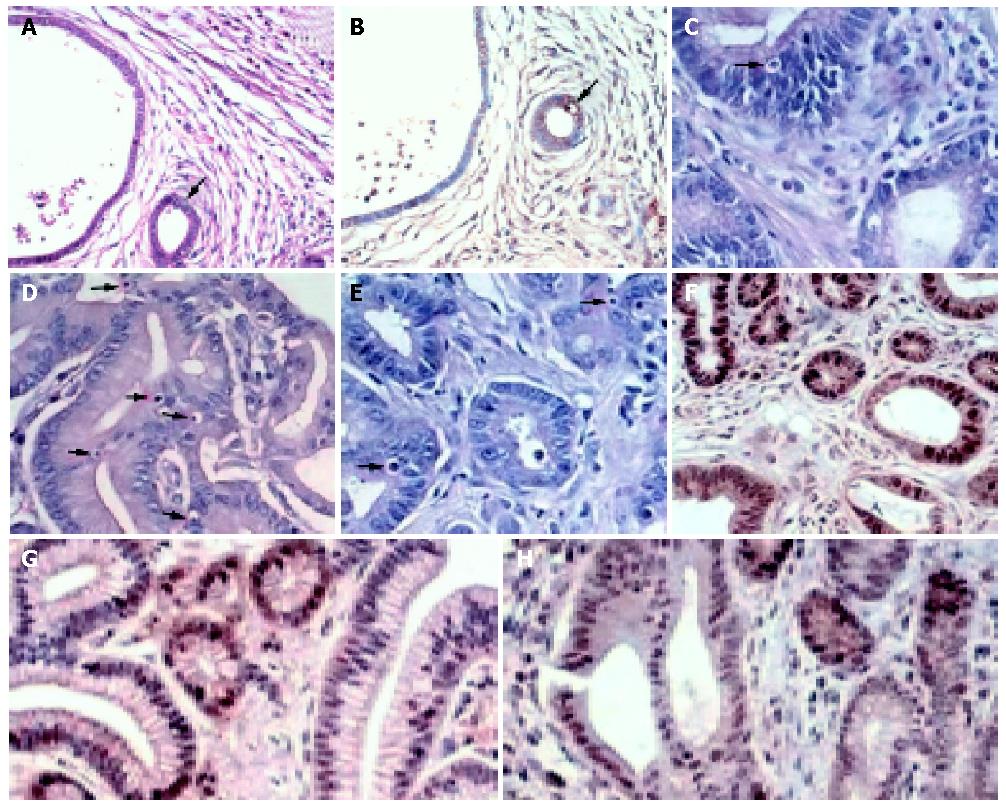
Apoptosis was determined by apoptotic nuclei counting. Sections were stained with hematoxylin and eosin to evaluate the number of apoptotic cells per section. The criteria used to recognize apoptotic cells were: shrunk size, loss of contact with surrounding tissues (at times forming the classically described halo) and nuclear condensation as previously described[19]. At least 1000 cells were counted in five random fields and the percentage of cells with apoptotic features was then calculated (apoptotic index or AI). The apoptotic nuclei counts were compared with findings obtained by terminal deoxynucleotidy transferase (TdT)-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) technique (DeadEndTM Colorimetric TUNEL System; Promega, Madison, WI, USA) in 30 randomly selected cases (Figures 1A, B). A strong correlation between apoptotic nuclei count and TUNEL results was found (r = 0.86, P<0.001).
Proliferation was assayed by immunoperoxidase staining for Ki-67 as described previously[19]. Briefly, paraffin-embedded sections from each specimen were labeled with anti-Ki-67 antibody (ab833; abcam, Cambridge, UK) after microwave antigen retrieval in citrate buffer. Negative controls were run by replacing the primary antibody with non-immune serum. The slides were developed in 3,3-diaminobenzidine tetrahydrochloride (DAB, Dako, Denmark) and counter-stained with Mayer haematoxylin (Figure 1). The proliferation index (PI) was expressed as a percentage of the ratio of Ki-67-positive nuclei to the total nuclei counted.
Results were expressed as mean±SE. Comparisons among different treatment groups were made by (analysis of variance) ANOVA with Bonferroni’s multiple comparison tests. P<0.05 was considered statistically significant. All statistical calculations were carried out using the SPSS statistical software package (version 11.0, SPSS Inc.).
The percentage of rats that developed gastric cancer in each treatment group is summarized in Table 1. Whilst none of the control rats in group A developed gastric cancer, 75% of MNNG treated rats (group B) had gastric cancer (P = 0.002, Table 1). Treatment with celecoxib at 10 mg/(kg·d) (18.8%, P = 0.004) and 20 mg/(kg·d) (31.3%, P = 0.052) was associated with lower incidences of gastric cancer development than MNNG control. However, administration of celecoxib 5 mg/kg or indomethacin 3 mg/kg had no significant reduction in tumor incidence.
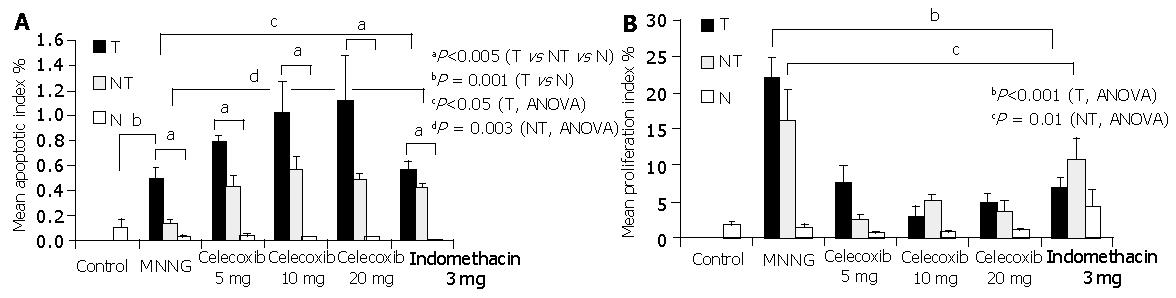
The mean apoptotic indexes in gastric tumors, their corresponding adjacent normal tissues and non-tumor gastric tissues of different treatment groups are shown in Figure 2A. The apoptotic index was generally higher in gastric tumors than in their adjacent non-tumor and normal gastric tissues (P<0.005, ANOVA). Specifically, there was a significant difference in the apoptotic indexes among tumor, adjacent non-cancer tissues and normal gastric tissues in group B as MNNG control (P = 0.001), groups C to E treated with celecoxib (P<0.005) and group F treated with indomethacin (P = 0.003).
Whilst the mean apoptotic index was 0.50% in MNNG treated tumors, there appeared to be a dose-dependent increase in the apoptotic index of gastric tumors treated with celecoxib (P<0.05, ANOVA). The corresponding mean tumor apoptotic indexes in rats treated with celecoxib 5 mg/(kg·d), celecoxib 10 mg/(kg·d) and celecoxib 20 mg/(kg·d) were 0.78% (P = 0.015 vs group B), 1.02% (P = 0.041 vs group B) and 1.12% (P = 0.093 vs group B), respectively. In contrast, indomethacin failed to induce apoptosis in gastric tumor (0.57% vs 0.50%, P = 0.54). Moreover, there was a significant difference in apoptotic indexes in the adjacent non-tumor tissues among different treatment groups (P = 0.003, ANOVA). The apoptotic index in non-tumor tissues increased from 0.13% in group B MNNG to 0.43% in celecoxib 5 mg/(kg·d) (P = 0.009), 0.56% in celecoxib 10 mg/(kg·d) (P = 0.01), 0.48% in celecoxib 20 mg/(kg·d) groups (P<0.001) and 0.42% in indomethacin group (P<0.05), respectively. On the other hand, the apoptotic index in the normal stomachs of non-tumor rats was low and comparable among different treatment groups (P = 0.39).
The highest proliferation index (22.1%) was seen in gastric tumors of MNNG-treated rats. Treatment with either celecoxib or indomethacin significantly reduced the tumor proliferation index (P<0.001, ANOVA; Figure 2B). The corresponding proliferation index in tumors treated with celecoxib 5, 10 and 20 mg/(kg·d) was 7.6% (P<0.001 vs group B), 2.9% (P = 0.012 vs group B) and 4.6% (P<0.001 vs group B) respectively. Celecoxib at 5, 10 and 20 mg/(kg·d) inhibited tumor proliferation by 65.6%, 86.9% and 79.2% respectively. Notably, the maximal anti-proliferative effect was achieved with celecoxib treatment at 10 mg/(kg·d). In contrast to apoptosis, similar anti-proliferative effects were noted in indomethacin-treated tumors (68.8% reduction, P<0.001 vs group B).
In adjacent normal tissues, there was also a significant difference in the proliferation indexes among different treatment groups (P = 0.01, ANOVA). The highest proliferation index was found in the adjacent non-tumor tissues of group B MNNG treated rats (16.1%). The corresponding proliferation indexes in non-tumor tissues of rats treated with celecoxib 5 mg/(kg·d), 10 mg/(kg·d) and 20 mg/(kg·d) were 2.44% (or 85% reduction, P = 0.012 vs group B), 5.21% (or 67.6% reduction, P>0.05 vs group B) and 3.63% (77.5% reduction, P>0.05 vs group B). In contrast, there was no significant suppression of proliferation in non-tumor tissues of indomethacin group (10.7%, P>0.05 vs group B). It was interesting to note that the proliferation of non-tumor gastric tissues appeared to be higher in indomethacin group than in those treated with celecoxib or MNNG control.
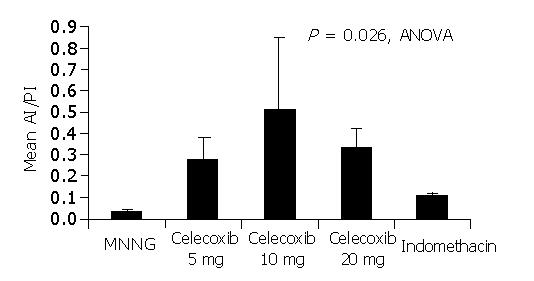
We also analyzed the ratio of apoptotic index to proliferation index (AI/PI) in gastric tumors of different treatment groups. As shown in Figure 3, there was a significant difference in the ratio among different treatment groups (P = 0.026, ANOVA). As shown in Table1, the AI/PI ratio was found to be inversely proportional to the tumor incidences of different treatment groups. The lowest AI/PI ratio (0.03±0.012) was seen in group B MNNG-treated tumors which had the highest tumor incidence (75%). In contrast, the highest AI/PI ratio (0.51±0.34) was seen in rats treated with celecoxib at 10 mg/(kg·d) (Group D) with the lowest tumor incidence (18.8%).
We have demonstrated in our recent study[16] that treatment with celecoxib, but not indomethacin could significantly reduce the number of gastric tumor formations in rats and the maximal chemopreventive effect was seen in rats treated with a moderate dose of celecoxib 10 mg/(kg·d). Intriguingly, the lowest COX-2 and PGE2 levels were detected in indomethacin-treated tumors but not in celecoxib-treated groups, suggesting that the chemopreventive effect may not be mediated by COX-2 or PGE2 suppression alone. This study aimed to characterize the cell kinetic changes in stomachs of rats after treatment with celecoxib or indomethacin in order to gain more insights into the mechanisms underlying the chemopreventive effects of celecoxib. We found that treatment with celecoxib at all doses 5-20 mg/(kg·d) or indomethacin caused a marked inhibition of proliferation in gastric tumors and their adjacent normal tissues. On the other hand, it was noted that induction of apoptosis was only noticed in celecoxib-treated tumors but not in indomethacin-treated tumors. Together, celecoxib treatment resulted in both induction of apoptosis and inhibition of proliferation. In contrast, indomethacin was found to inhibit cell proliferation without induction of apoptosis in gastric tumors. These findings suggest that the mechanisms underlying the chemopreventive effect of celecoxib may be more related to its ability to induce apoptosis which was not found in indomethacin-treated group. More importantly, these findings help to explain the divergent chemopreventive responses of rat stomachs to these two agents which could not be explained by the level of COX-2 inhibition alone.
Although there was no induction of apoptosis by indomethacin in gastric tumors, we noticed that both indomethacin and celecoxib induced apoptosis in adjacent normal gastric tissues. The reason for this discrepant finding remains elusive but it is possible that neoplastic transformation of gastric epithelial cells may render them less susceptible to the pro-apoptoic effects of indomethacin. Intuitively, the use of a higher dose of indomethacin might be able to induce apoptosis in gastric tumor cells. The use of this dosage of 3 mg/(kg·d) is supported by previous animal chemopreventive studies[10,11] and human daily recommendations. Moreover, results from our previous study[16] provide unequivocal evidence that the current dosage is adequate in suppressing COX-2 and PGE2. Future studies may be necessary to characterize the effects of a high dose of indomethacin in gastric cancer chemoprevention. However, the use of a higher dosage may result in more gastrointestinal toxicity as reflected by the heightened proliferation in non-tumor tissues treated with the current dose of indomethacin (Figure 2B). This increase in gastric proliferation may be a compensatory response to the topical erosive effect of non-selective NSAIDs.
Moreover, the current study helps to explain the optimal dose of celecoxib used in chemoprevention of gastric cancer. As shown in Figure 3, treatment with celecoxib at 10 mg/(kg·d) was associated with the highest AI/PI ratio. Although we have shown in our previous study[16] that the high dose celecoxib 20 mg/(kg·d) is associated with greater suppression of COX-2 activity and PGE2 level, this is not associated with a parallel rise in AI/PI ratio and higher chemopreventive effects. In line with our findings, Nishimura et al[20] reported that induction of apoptosis was noted after treatment with a COX-2 inhibitor at a lower concentration than for the suppression of cell proliferation in a cancer xenograft model. It thus appears that the optimal dosage of celecoxib in chemoprevention is the dosage with the highest apoptosis to proliferation ratio.
Apart from suppression of prostaglandins, other possible pathways by which COX-2 inhibitors exert their pro-apoptotic effects have been previously addressed. It has been shown that NS398, a specific COX-2 inhibitor, could induce apoptosis in COX-2 expressing esophageal cancer cell line through the cytochrome C-dependent pathway with activation of Caspase-9 and Caspase-3[21]. This is associated with minimal alterations in bcl-2, bax, c-myc, Fas and Fas-ligand expressions. A recent study also showed that celecoxib could induce apoptosis via a novel apoptosome-dependent but Bcl-2-independent mitochondrial pathway[22]. Both Fas-associated death domain protein and Bcl-2 are not involved in the induction of apoptosis by celecoxib in Jurkat T cells. This effect also appears to be independent of the ability to block COX-2. In addition, the failure of indomethacin to inhibit the development of MNNG-induced gastric cancer may be explained by the inability of indomethacin to inhibit the activity of IκB kinase β[23]. The NF-κB signaling pathway is another potential non-COX mediated-carcinogenesis pathway[24]. Activated NF-κB could translocate into the nuclei where it modulates the expression of a variety of genes, mostly through IκB kinase (IKK)-dependent phosphorylation and subsequent degradation of its inhibitors. It has been recognized that aspirin and sulindac, but not indomethacin, can inhibit the activity of IκB kinase βin vitro. Therefore, the failure of indomethacin to inhibit IκB kinase β may result in less COX-independent tumor suppression. Whether the difference in IκB kinase β inhibitory effects accounts for the differences in outcomes between indomethacin and celecoxib warrants further investigation.
In conclusion, these data help to explain the divergent chemopreventive effects of celecoxib and indomthacin in this animal model of gastric carcinogenesis. The chemopreventive effect of celecoxib is largely mediated by induction of apoptosis through a probable COX2-independent pathway. Further studies are necessary to characterize the pathways involved and the possible role of celecoxib in chemoprevention of human gastric cancer.
Edited by Wang XL
| 1. | Langman MJ, Cheng KK, Gilman EA, Lancashire RJ. Effect of anti-inflammatory drugs on overall risk of common cancer: case-control study in general practice research database. BMJ. 2000;320:1642-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 309] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 2. | Akre K, Ekström AM, Signorello LB, Hansson LE, Nyrén O. Aspirin and risk for gastric cancer: a population-based case-control study in Sweden. Br J Cancer. 2001;84:965-968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 128] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Wang WH, Huang JQ, Zheng GF, Lam SK, Karlberg J, Wong BC. Non-steroidal anti-inflammatory drug use and the risk of gastric cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2003;95:1784-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 201] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 4. | Patrignani P. Nonsteroidal anti-inflammatory drugs, COX-2 and colorectal cancer. Toxicol Lett. 2000;112-113:493-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Vane JR, Botting RM. Mechanism of action of antiinflammatory drugs. Int J Tissue React. 1998;20:3-15. [PubMed] |
| 6. | Sung JJ, Leung WK, Go MY, To KF, Cheng AS, Ng EK, Chan FK. Cyclooxygenase-2 expression in Helicobacter pylori-associated premalignant and malignant gastric lesions. Am J Pathol. 2000;157:729-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 197] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Leung WK, To KF, Ng YP, Lee TL, Lau JY, Chan FK, Ng EK, Chung SC, Sung JJ. Association between cyclo-oxygenase-2 overexpression and missense p53 mutations in gastric cancer. Br J Cancer. 2001;84:335-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Pairet M, Engelhardt G. Distinct isoforms (COX-1 and COX-2) of cyclooxygenase: possible physiological and therapeutic implications. Fundam Clin Pharmacol. 1996;10:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 140] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908-7916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1028] [Cited by in RCA: 1053] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 10. | Sawaoka H, Kawano S, Tsuji S, Tsujii M, Gunawan ES, Takei Y, Nagano K, Hori M. Cyclooxygenase-2 inhibitors suppress the growth of gastric cancer xenografts via induction of apoptosis in nude mice. Am J Physiol. 1998;274:G1061-G1067. [PubMed] |
| 11. | Charalambous D, O'Brien PE. Inhibition of colon cancer precursors in the rat by sulindac sulphone is not dependent on inhibition of prostaglandin synthesis. J Gastroenterol Hepatol. 1996;11:307-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Elder DJ, Halton DE, Hague A, Paraskeva C. Induction of apoptotic cell death in human colorectal carcinoma cell lines by a cyclooxygenase-2 (COX-2)-selective nonsteroidal anti-inflammatory drug: independence from COX-2 protein expression. Clin Cancer Res. 1997;3:1679-1683. [PubMed] |
| 13. | Hanif R, Pittas A, Feng Y, Koutsos MI, Qiao L, Staiano-Coico L, Shiff SI, Rigas B. Effects of nonsteroidal anti-inflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem Pharmacol. 1996;52:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 458] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 14. | Chan TA. Nonsteroidal anti-inflammatory drugs, apoptosis, and colon-cancer chemoprevention. Lancet Oncol. 2002;3:166-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 174] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 15. | Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, McKeown-Eyssen G, Summers RW, Rothstein R, Burke CA. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1021] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 16. | Hu PJ, Yu J, Zeng ZR, Leung WK, Lin HL, Tang BD, Bai AH, Sung JJ. Chemoprevention of gastric cancer by celecoxib in rats. Gut. 2004;53:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Sugimura T, Fujimura S. Tumour production in glandular stomach of rat by N-methyl-N'-nitro-N-nitrosoguanidine. Nature. 1967;216:943-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 227] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Tatematsu M, Ozaki K, Mutai M, Shichino Y, Furihata C, Ito N. Enhancing effects of various gastric carcinogens on development of pepsinogen-altered pyloric glands in rats. Carcinogenesis. 1990;11:1975-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Yu J, Leung WK, Go MY, Chan MC, To KF, Ng EK, Chan FK, Ling TK, Chung SC, Sung JJ. Relationship between Helicobacter pylori babA2 status with gastric epithelial cell turnover and premalignant gastric lesions. Gut. 2002;51:480-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Nishimura G, Yanoma S, Mizuno H, Kawakami K, Tsukuda M. A selective cyclooxygenase-2 inhibitor suppresses tumor growth in nude mouse xenografted with human head and neck squamous carcinoma cells. Jpn J Cancer Res. 1999;90:1152-1162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Li M, Wu X, Xu XC. Induction of apoptosis by cyclo-oxygenase-2 inhibitor NS398 through a cytochrome C-dependent pathway in esophageal cancer cells. Int J Cancer. 2001;93:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Jendrossek V, Handrick R, Belka C. Celecoxib activates a novel mitochondrial apoptosis signaling pathway. FASEB J. 2003;17:1547-1549. [PubMed] |
| 23. | Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998;396:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1219] [Cited by in RCA: 1240] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 24. | Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1908] [Cited by in RCA: 1954] [Article Influence: 85.0] [Reference Citation Analysis (0)] |