Published online Jan 7, 2005. doi: 10.3748/wjg.v11.i1.17
Revised: March 21, 2004
Accepted: April 13, 2004
Published online: January 7, 2005
AIM: To answer the question whether FHIT gene expression is affected by the family history of gastric carcinoma and the presence of Helicobacter pylori (H pylori) in the gastric mucosa of patients with dyspepsia.
METHODS: FHIT gene expression in two different topographic sites of the gastric mucosa of twenty-one patients with dyspepsia and with or without familial gastric carcinoma, infected or not infected with H pylori, was evaluated by reverse transcription-PCR (RT-PCR) and IMAGE QUANT methods. A rapid urease test and histopathological examination were used to determine H pylori colonization.
RESULTS: In the gastric mucosa of patients with family histories of gastric carcinoma, the amount of FHIT protein mRNA was reduced down to 32%, and for patients with H pylori colonization, to 24% in comparison to controls with dyspepsia and without cancer in the family. FHIT expression was independent of the topography of specimens (corpus vs antrum), and for the control patients it was less sensitive to infection with H pylori. A considerable statistical difference in FHIT levels was observed in the gastric mucosa from the corpus of patients with family histories of gastric carcinoma in respect to H pylori colonization (P = 0.06). Macroscopic evaluation of the gastric mucosa demonstrated that pathologic changes classified according to the Sydney system had no significant influence on FHIT expression within each tested group of patients.
CONCLUSION: Loss of FHIT expression was observed in patients with dyspepsia and family histories of gastric carcinoma, especially those infected with H pylori. Such results may constitute an early indication of the development of gastric carcinoma, which is associated with family factors including heredity and H pylori infection. The loss of the FHIT gene may serve as a marker for early diagnosis and prevention of gastric carcinoma, especially in context of early monitoring of H pylori infection in individuals with a record of familial stomach cancer.
-
Citation: Stec-Michalska K, Antoszczyk S, Klupinska G, Nawrot B. Loss of
FHIT expression in gastric mucosa of patients with family histories of gastric cancer andHelicobacter pylori infection. World J Gastroenterol 2005; 11(1): 17-21 - URL: https://www.wjgnet.com/1007-9327/full/v11/i1/17.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i1.17
Poland is among countries with high risk of gastric carcinoma. In both men and women it constitutes the second cause of death, after lung cancer[1]. The incidence of this tumor, irrespective of great differences in its prevalence in particular geographic regions, depends on socio-economic structure, eating habits, age, gender, and profession. In Poland, the incidence rate of gastric carcinoma is approximately 19.7 per 100000 men and 7 per 100000 women. Incidence and mortality increase with age. Recently, the 5-year survival rate of patients with gastric carcinoma in Poland has increased to 25.9%[2]. The most prevalent malignant tumor of the stomach is adenocarcinoma (93%). The others are lymphomas (5%), mesenchymal tumors and carcinoids (1%).
Etiopathogenesis of gastric carcinoma cannot disregard the evidence that familial and hereditary factors increase individual susceptibility, especially in those who are exposed to environmental hazards. Such hazards include Helicobacter pylori (H pylori) infection. A comparison of gastric carcinoma incidence with that of H pylori infection implies that (except for African continent where despite the infection, cancers are less prevalent) it is higher in developing countries, where infections are highly prevalent. This suggests that a bacterial factor is involved in the pathogenesis of gastric carcinoma. In Poland the percentage of H pylori infection is high, reaching approximately 40-60%, while in developed countries it reaches approximately 20-40%.
The genetic contribution to gastric carcinoma is not yet clear. Literature data indicate that, as in many other tumors, the fragile histidine triad (FHIT) protein occurs at very low concentrations or is completely lost in most specimens from stomach tumors[3,4]. Accordingly, inactivation of the FHIT gene may predispose the development of cancer[5]. Several studies indicate that the anticancer effects of FHIT protein are due to the induction of apoptosis. Thus it has not been possible to estimate whether the loss of FHIT gene expression is a cause or a consequence of the development of cancer, or whether it is a primary or secondary event. As demonstrated in in vivo studies, re-expression of the FHIT gene (through gene therapy) reverses the development of established tumors by 60-70% through an apoptotic pathway[6,7].
Here we present our preliminary results on FHIT expression in the gastric mucosa of patients with dyspepsia and with or without familial histories of gastric carcinoma. The impact of H pylori infection on those patients has also been studied.
Selection of patients A group of 21 dyspepsia patients, aged below 60, was screened in these studies. They were divided into two groups. Group I consisted of 11 subjects without family histories of neoplasms, including 5 patients infected with H pylori. Group II consisted of 10 patients with family histories of gastric carcinoma, 5 of them were infected with H pylori. A routine rapid urease test for the presence of H pylori was used for the selection of infected patients. For at least 14 d before the examination, the patients did not take any H2 blockers and proton pump inhibitors.
In endoscopic biopsies from the upper digestive tract (antrum and corpus), 4 specimens were taken from each patient for pathomorphological evaluation and colonization of H pylori (two for the rapid urease test and two for histopathological examination) and 4 specimens from identical sites to evaluate the expression of the FHIT gene. Biopsies were taken routinely, using a gastrofibroscope GIF Q140 or GIF Q145 (Olympus, Tokyo, Japan).
The gastric mucosal specimens were collected with sterile forceps, four from the antrum (3-5 cm proximally from the pylorus) and four from the corpus (5-8 cm distally from the cardia). For histopathological evaluation of the H pylori colonization, the specimens from the corpus and antrum were loaded into 1% formalin and routinely screened with microscope (Giemsay method). Each specimen for FHIT evaluation was rinsed three times with PBS buffer without ions Ca2+ and Mg2+, treated with 1 mL of lysing reagent - TriPure isolation reagent (Boehringer Mannheim) and homogenized. Tissue lysates could be kept at -70 °C for a maximum of 2-4 wk.
Macroscopic evaluation of the gastric mucosa Macroscopic evaluation of the gastric mucosa was based on the 4-degree Sydney modified classification system[8] , i.e.,: (1) lack of evident changes or focal hyperaemia of the mucosa; (2) erythematous-edematous changes in the antrum; (3) erythematous-edematous changes with single erosions in the corpus and antrum; (4) diffuse erythematous-edematous changes in the whole stomach, with haemorrhagic extravasations and flat or convex erosions or intestinal metaplasia foci.
Isolation of total RNA from gastric tissue The total RNA fraction was isolated from the tissue lysates according to the TriPure Isolation Reagent protocol. The nucleic acid fraction was then treated with RQ1 RNase-free DNase (Promega) and isolated by phenol/chloroform extraction followed by ethanol precipitation. The total RNA was quantified spectrophotometrically at 260 nm. Samples could be kept at -70 °C for several months without any decomposition of the RNA.
Determination of the level of FHIT mRNA in tissue lysates The level of FHIT mRNA was monitored by a semi-quantitative RT-PCR method using a OneStep RT-PCR kit (Qiagen, Germany). The specific FHIT primers (1 μL each) at a concentration of 20 μmol/L were used to give an RT-PCR product of 507 nucleotides long. RT primer (5’- CCT GCG TCC TGA TGA AGT GG-3’, P1FHIT), PCR primer (5’-TGCCTGTCTGAGCCGTTTAG-3’, P2FHIT) and a total RNA (0.5 μg) were used for the RT-PCR reaction (50 μL volume). PCR was programmed for 30 cycles. The reaction product was analysed by 3% NuSieve GTG agarose (FMC BioProducts, Rockland, ME, USA) gel electrophoresis and stained with ethidium bromide. An amplification of a house-keeping GAPDH gene with specific primers P1GAPDH (5’-CATCATCTCTGCCCCCTCTG-3’) and P2GAPDH (5’-TCCACGATACCAAAGTTGTC-3’) (1 μL each) at a concentration of 20 μmol/L and 0.5 μg of total RNA was used as a control to give the desired 150 bp product. The level of mRNA of FHIT protein is expressed as a ratio of FHIT to GAPDH amplification products (FHIT/GAPDH). Quantification of gels was done with the IMAGE QUANT computer program. For each set of data average weight and SEM were calculated.
Data of FHIT are expressed as mean±SE. For statistical analysis of a difference between mean values of FHIT, we used Student (t) or chi-square (χ2) tests, depending on the extent of the variance. The statistical significance of this difference was identified for each test by a two-tailed probability (P). P values less than 0.05 were considered statistically significant.
The group II patients were selected from those exhibiting dyspepsia and with family histories of stomach cancer in the first-degree relatives and with cancers of other organs in the first- or second-degree relatives. Patients with similar dyspepsia symptoms but without familial cancer were selected as control subjects (group I). Macroscopic evaluation of the gastric mucosa was based on the 4-degree modified Sydney classification[8] (see Materials and Methods). The characteristics of the test patients are given in Table 1.
| Selection criteria | Sex / Hp(+) | Average age (yr) | Sydney system | ||||
| Female | Male | I | II | III | IV | ||
| Group I (no familiar cancer) | 7/3 | 4/2 | 46.3 | 3 | 5 | 2 | 1 |
| Group II (stomach cancer in first-degree relatives and cancer of other organs in first- or second- degree relatives) | 6/3 | 4/2 | 47.4 | 1 | 1 | 2 | 6 |
All patients were screened in a urease test for the presence of H pylori. In addition, histopathological examination of tissue samples from the gastric mucosa of the antrum and corpus was carried out for each individual subject. Eight patients in both tests showed positive H pylori (+) infection, while two patients showed negative urease tests but positive histopathological findings for the presence of H pylori. Those patients were included in the H pylori (+) group.
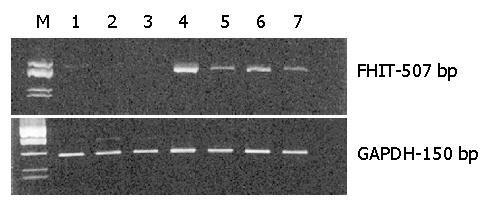
Specimens taken from the antrum and corpus of each patient were lysed and the total RNA was isolated. The level of FHIT expression was determined by a semi-quantitative reverse transcription and PCR (RT-PCR) method with FHIT gene specific primers. Amplification products were analyzed by 3% agarose gel electrophoresis. Representative electrophoretic analyses of the RT-PCR products of FHIT (upper gel) and of a control GAPDH (lower gel) are shown in Figure 1. Lanes 1-3 represent three patients of group II with family histories of gastric carcinoma and with H pylori infection. Analysis of specimens from the corpus of those patients shows very little or no FHIT expression. The remaining lanes represent individuals of group I with negative (lane 4) and positive H pylori infection (lanes 5-7). For patients with no familial cancer, the level of FHIT expression is higher than in those with cancer in the family (lanes 4-7 vs 1-3). Moreover, for group I patients the FHIT expression is significantly affected by H pylori colonization (compare line 4-Hp (-) with lanes 5-7-(Hp(+)).
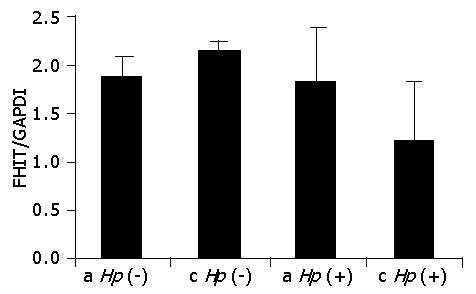
Comparison of the FHIT expression profile was made for all patients of both tested groups. RT-PCR products were quantified by densitometry with the IMAGE QUANT program and the ratio of FHIT to GAPDH (FHIT/GAPDH) was determined for each case. Figure 2 shows comparison of the mean values of FHIT expression in the gastric mucosa of group I patients relative to the topography of the biopsied gastric specimens and the colonization of H pylori. No significant differences between FHIT values for the antrum and corpus in both Hp (-) (1.86 vs 2.16) and Hp (+) individuals (1.82 vs 1.22). Also, there were no statistical differences in the FHIT level within the same topographic parts caused by bacterial infection. Thus, for patients with dyspepsia and with no familial cancer, the FHIT expression level was independent of both the topography of the specimens and the bacterial infection.
Comparison of the mean values of FHIT expression in the gastric mucosa of group II patients with the topography of the biopsied gastric specimens and colonization of H pylori is shown in Figure 3. No significant difference between FHIT values for the antrum versus the corpus was observed in both Hp (-) (0.61 vs 0.69) and Hp (+) individuals (0.47 vs 0.27). There was also no statistical difference in FHIT level within the antrum caused by bacterial infection. However, considerable statistical difference was observed for the FHIT level in the corpus depending on H pylori colonization (P = 0.06). Thus, for patients with family histories of gastric cancer, the FHIT expression level was independent of the topography of the biopsied specimens but it was significantly affected by the presence of H pylori in the stomach corpus.
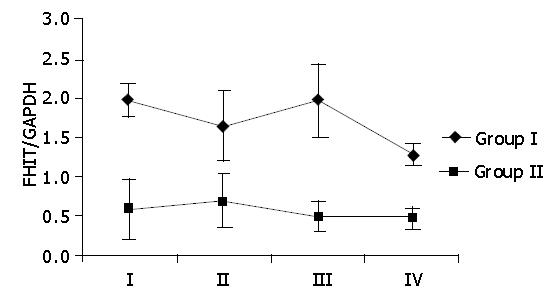
The FHIT/GAPDH mean values for the patients of groups I and II were compared with the macroscopic evaluation of the gastric mucosa based on the 4-degree Sydney system[8]. The FHIT/GAPDH mean values were calculated for all cases of each Sydney system group, independent of stomach topography and colonization with H pylori. Macroscopic evaluation of the gastric mucosa showed no significant differences in the FHIT level within each tested group of patients (Figure 4). Statistical data are as follows: for patients of group I χ2 = 2.51 and P>0.1 and for patients of group II χ2 = 1.78 and P>0.1.
The origin of tumors is connected with a lack of balance between the proliferation of cells and their removal through apoptosis. Most frequently, in neoplastic transformation, the mechanism of apoptosis fails and cellular proliferation increases. This process is complex and consists of many stages. The loss of the FHIT gene function is frequent in neoplastic transformation; it may determine the origin of cancer.
Inactivation of the FHIT gene has been observed in tissues collected from tumors of many organs, including head, neck, breasts, lungs, pancreas, stomach, ovary, colorectum, bladder, and also in leukemia[3,9,10]. An impairment of FHIT gene transcription has also been demonstrated in 86% of patients with diagnosed Barett’s metaplasia[11] and in 93% of patients with esophageal adenocarcinoma[12]. According to the results of Skopelitou et al[13], FHIT protein is absent in 79% of tested tissue material in specimens from gastric mucosa of biopsied adenocarcinoma, affected by H pylori. The contribution of this bacterium to neoplastic transformation is well documented. H pylori causes an increase in proliferative activity and affects the apoptotic process of the glandular epithelium of gastric mucosa. The sequence of consecutive steps of pathological lesions as a result of H pylori infection, i.e., chronic atrophic inflammation of gastric mucosa → intestinal metaplasia → dysplasia → gastric carcinoma, may last for years[14,15]. Atrophic lesions in gastric mucosa usually refer to the antrum, seldom to the corpus. Cancer develops mainly in the antrum (70%), although in recent years its topography has been noticed to shift towards the corpus. This is associated with a higher frequency of H pylori in that area[16].
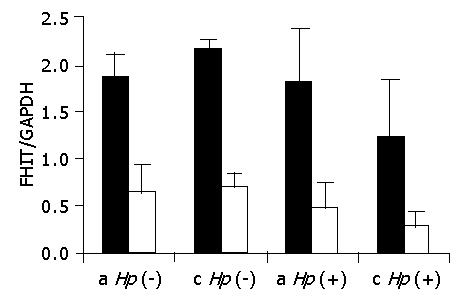
We ask the question whether loss of the FHIT gene occurs only after development of gastric adenocarcinoma or if its expression is affected by other factors which may lead to the early neoplastic transformation. We have considered the influence of familial factors including the heredity of gastric cancer and H pylori infection. We have compared FHIT expression in patients (below the age of 60) with dyspepsia, family histories of stomach cancer in the first-degree relatives, and cancer of other organs in the first- or second- degree relatives, including patients infected with H pylori (group II) against control patients without family histories of gastric carcinoma (group I). Although the analyzed group was small, we observed a significant loss of FHIT (68%) in patients with negative H pylori (group II versus group I) (Figure 5). The FHIT mean values for the antrum were 0.61 and 1.86 for groups II and I (P<0.05), while for the corpus they were 0.69 and 2.16 (P<0.05), respectively. Infection with H pylori caused loss of FHIT in both tested groups of patients. In the antrum the mean values of FHIT were 0.47 and 1.82 for patients of group II and group I, respectively, with a P value of 0.057. The loss of FHIT in the corpus of patients with positive H pylori and familial cancer did not reach statistical significance (P>0.1) in comparison with the Hp (+) group I patients since FHIT expression was significantly decreased in this part of the stomach by the bacterial infection itself. This effect was observed in earlier studies[16] where it was reported that despite the development of cancer mainly in the antrum, the topography of cancer shifted towards the corpus, assisted by the higher frequency of bacterial colonization in that area.
Lower FHIT expression in the gastric mucosa infected with H pylori suggests that bacterial colonization affects the metabolic pathway and interferes with FHIT expression in patients of both tested groups. The loss of FHIT observed in the patients with dyspepsia may constitute an early indication of the development of gastric carcinoma. These results may help understand the role of FHIT protein in the process of carcinogenesis, and its function in individuals with familial gastric carcinoma and H pylori infection.
The studies on the evaluation of the expression of FHIT protein at the mRNA level are encouraging, but only a complex evaluation of the tissue material from specific parts of the stomach, determining the level of FHIT expression, concentrations of FHIT protein, and the extent of infection and pathogenicity of H pylori strains (presence of Cag A protein gene)[17-19], will allow verification of the research hypothesis proposed in this paper. Further studies should answer the question whether it is necessary to monitor people with family histories of gastric carcinoma, especially those infected with H pylori.
We should emphasize that so far, no studies have been carried out to determine the level of FHIT expression in the gastric mucosa of those with family histories of gastric cancer. The importance of family factors (including heredity) may be proved by the occurrence of gastric carcinoma in monozygotic twins[20] and a much higher prevalence of gastric carcinoma in certain families over several consecutive generations[21]. The hazards of early exposure of these family members to H pylori bacteria cannot be overestimated.
In conclusion, the significant decrease of FHIT expression observed in patients with dyspepsia and family histories of gastric carcinoma may indicate the need for monitoring the development of gastric carcinoma. The loss of the FHIT gene may serve as a marker for early diagnosis and prevention of gastric carcinoma. The possible manipulation of FHIT cellular activity, including gene therapy[6,7,22,23], constitutes a challenge for further studies aimed at the development of new therapeutic procedures for stomach cancer prevention.
The authors thank Professor Jan Chojnacki for scientific support and critical reading of the manuscript and Dr. Magdalena Janicka for statistical evaluation of the data.
Edited by Wang XL and Ma JY
| 1. | Wronkowski Z, Zwierko M, Chmielarczyk W. Epidemiology of malingant tumors in Poland. Przewodnik lekarza. 2000;12-14. |
| 2. | Popiela T, Kulig J. Multi-theraphy as a chance for improvement of a treatment of gastric carcinoma in Poland. Nowotwory. 1996;46:28-74. |
| 3. | Baffa R, Veronese ML, Santoro R, Mandes B, Palazzo JP, Rugge M, Santoro E, Croce CM, Huebner K. Loss of FHIT expression in gastric carcinoma. Cancer Res. 1998;58:4708-4714. [PubMed] |
| 4. | Lee SH, Kim WH, Kim HK, Woo KM, Nam HS, Kim HS, Kim JG, Cho MH. Altered expression of the fragile histidine triad gene in primary gastric adenocarcinomas. Biochem Biophys Res Commun. 2001;284:850-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Ishii H, Ozawa K, Furukawa Y. Alteration of the fragile histidine triad gene early in carcinogenesis: an update. J Exp Ther Oncol. 2003;3:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Ishii H, Zanesi N, Vecchione A, Trapasso F, Yendamuri S, Sarti M, Baffa R, During MJ, Huebner K, Fong LY. Regression of upper gastric cancer in mice by FHIT gene delivery. FASEB J. 2003;17:1768-1770. [PubMed] |
| 7. | Siprashvili Z, Sozzi G, Barnes LD, McCue P, Robinson AK, Eryomin V, Sard L, Tagliabue E, Greco A, Fusetti L. Replacement of Fhit in cancer cells suppresses tumorigenicity. Proc Natl Acad Sci USA. 1997;94:13771-13776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 265] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3550] [Article Influence: 122.4] [Reference Citation Analysis (3)] |
| 9. | Pekarsky Y, Zanesi N, Palamarchuk A, Huebner K, Croce CM. FHIT: from gene discovery to cancer treatment and prevention. Lancet Oncol. 2002;3:748-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Zhao P, Song X, Nin YY, Lu YL, Li XH. Loss of fragile histidine triad protein in human hepatocellular carcinoma. World J Gastroenterol. 2003;9:1216-1219. [PubMed] |
| 11. | Michael D, Beer DG, Wilke CW, Miller DE, Glover TW. Frequent deletions of FHIT and FRA3B in Barrett's metaplasia and esophageal adenocarcinomas. Oncogene. 1997;15:1653-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Mori M, Mimori K, Shiraishi T, Alder H, Inoue H, Tanaka Y, Sugimachi K, Huebner K, Croce CM. Altered expression of Fhit in carcinoma and precarcinomatous lesions of the esophagus. Cancer Res. 2000;60:1177-1182. [PubMed] |
| 13. | Skopelitou AS, Mitselou A, Katsanos KH, Alexopoulou V, Tsianos EV. Immunohistochemical expression of Fhit protein in Helicobacter pylori related chronic gastritis, gastric precancerous lesions and gastric carcinoma: correlation with conventional clinicopathologic parameters. Eur J Gastroenterol Hepatol. 2003;15:515-523. [PubMed] |
| 14. | Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol. 1995;19 Suppl 1:S37-S43. [PubMed] |
| 15. | Lauwers GY. Defining the pathologic diagnosis of metaplasia, atrophy, dysplasia, and gastric adenocarcinoma. J Clin Gastroenterol. 2003;36:S37-S43; discussion S61-S62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Popiela T, Stachura J. Cancers of digestive tract. Gastroenterologia i Hepatologia Kliniczna, Konturek SJ. (Ed.). PZWL Warszawa. 2001;679-739. |
| 17. | Parsonnet J, Friedman GD, Orentreich N, Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997;40:297-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 672] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 18. | Konturek PC, Konturek SJ, Starzyska T, Marlicz K, Bielanski W, Pierzchalski P, Karczewska E, Hartwich A, Rembiasz K, Lawniczak M. Helicobacter pylori-gastrin link in MALT lymphoma. Aliment Pharmacol Ther. 2000;14:1311-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Takeuchi K, Ohno Y, Tsuzuki Y, Ando T, Sekihara M, Hara T, Kuwano H. Helicobacter pylori infection and early gastric cancer. J Clin Gastroenterol. 2003;36:321-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Verkasalo PK, Kaprio J, Koskenvuo M, Pukkala E. Genetic predisposition, environment and cancer incidence: a nationwide twin study in Finland, 1976-1995. Int J Cancer. 1999;83:743-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Bakir T, Can G, Siviloglu C, Erkul S. Gastric cancer and other organ cancer history in the parents of patients with gastric cancer. Eur J Cancer Prev. 2003;12:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, Gore M, Ironside J, MacDougall RH, Heise C. a controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 761] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 23. | Ishii H, Dumon KR, Vecchione A, Trapasso F, Mimori K, Alder H, Mori M, Sozzi G, Baffa R, Huebner K. Effect of adenoviral transduction of the fragile histidine triad gene into esophageal cancer cells. Cancer Res. 2001;61:1578-1584. [PubMed] |