Published online Jan 7, 2005. doi: 10.3748/wjg.v11.i1.136
Revised: December 12, 2003
Accepted: January 9, 2004
Published online: January 7, 2005
AIM: Transforming growth factor-β (TGF-β) plays a regulatory role in tissue repair. In a previous study, we found that TGF-β and its receptors were expressed in gastric mucosa of patients with well-healed gastric ulcers, as demonstrated by immunohistochemistry. To further characterize the role of TGF-β and its receptors in repairing gastric ulcers, we investigated the expression patterns of TGF-β and its receptors in gastric mucosa by in situ hybridization and reverse transcriptase-polymerase chain reaction (RT-PCR).
METHODS: Seventy-four patients with endoscopically proven gastric ulcers were eligible for participation in this study. All patients had routine biopsies on initial endoscopy and were then treated for 12 wk with an H2 blocker. Repeat endoscopy was then performed. There were 8 patients with poorly healed ulcers, and biopsies were taken from the margin of the residual ulcers. These tissue samples, along with biopsy of gastric mucosa near the original ulcers from 8 randomly selected patients with well-healed ulcers were examined for TGF-β and TGF-β receptor II mRNA by RT-PCR and in situ hybridization, as well as immunohistochemistry.
RESULTS: TGF-β and TGF-β receptor II were strongly expressed in tissues from patients with well-healed ulcers. Four of the 8 patients with poor healing had low or absent expression of TGF-β or TGF-β receptor II mRNA. All cases positive by RT-PCR assay were confirmed by in situ hybridization as well as immunohistochemistry.
CONCLUSION: It is suggested that TGF-β and its receptors are important for gastric ulcer healing. These results may have implications for further investigation of the healing process and in predicting response to therapy.
- Citation: Shih SC, Tseng KW, Lin SC, Kao CR, Chou SY, Wang HY, Chang WH, Chu CH, Wang TE, Chien CL. Expression patterns of transforming growth factor-beta and its receptors in gastric mucosa of patients with refractory gastric ulcer. World J Gastroenterol 2005; 11(1): 136-141
- URL: https://www.wjgnet.com/1007-9327/full/v11/i1/136.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i1.136
Several lines of evidence indicate that a number of cytokines, including platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), and transforming growth factor-β (TGF-β), are involved in the natural history of ulcers. Among many cytokines involved in cell growth and differentiation, TGF-β is noteworthy because of its multiple functions in a variety of cells. TGF-β is believed to be essential in wound healing for regulation of cell growth and differentiation and is known to be involved in tissue repair and remodeling.
TGF-β mRNA expression is up-regulated after gastric ulcers are induced with acetic acid in rats[1]. Immunoreactive TGF-β can be found in epithelial cells beneath the proliferative zone, as well as in macrophages and fibroblasts or myofibroblasts of the granulation tissue[1]. This indicates that TGF-β expression is part of the normal healing response of the gastric tissue, but it is still unclear whether it exerts a positive or negative influence on the speed and quality of ulcer healing. Studies in rats treated with subserosal injection of neutralizing antibodies against TGF-β have shown that the healing of acetic acid-induced ulcers is accelerated, with reduced fibrotic residue, while treatment with TGF-β itself leads to excessive deposition of extracellular matrix and scarring[2]. The function of TGF-βin vivo in human gastric ulcer healing, however, is not fully understood.
TGF-β family members exert their effects through binding to specific cell surface receptors. Several different molecules have been shown to bind to TGF-βs. The biological activity of TGF-β1 is mediated through binding to a heteromeric complex of TGF-β receptors I and II[3,4].
In a previous study[5], we used immunohistochemistry to examine TGF-β and its receptors in the gastric mucosa of patients with gastric ulcers. We found that TGF-β and its receptors were abundant in the gastric tissue of patients with well-healed gastric ulcers. By contrast, patients with ulcers refractory to treatment with standard doses of an H2 blocker had very variable staining for TGF-β and/or its receptors. However, immunohistochemical staining is basically a qualitative test. In order to further quantify the expression of TGF-β and its receptors in the gastric mucosa of patients with gastric ulcers, we designed the present study, measuring the gene expression of these substances using in situ hybridization and reverse transcriptase-polymerase chain reaction (RT-PCR).
From July 2000 to June 2002, patients with spontaneous gastric ulcers demonstrated on upper gastrointestinal endoscopy were considered for enrollment in the study. As in our previous investigation[5], patients were excluded if they had used NSAIDs, or were smokers, or had already had partial treatment for their ulcer. With the patients’ consent, an endoscopic biopsy was taken from the margin of the ulcer in all patients and sent for routine pathology. The patients were then treated with a standard regimen of either ranitidine or nizatidine 300 mg daily for 12 wk. After treatment was completed, an endoscopy was performed again to evaluate the results. Repeat biopsies were taken from the ulcer margin in patients with unhealed ulcers and from the area near the original ulcer in patients whose ulcer had healed well. At least 6 pieces of gastric tissue were obtained during each biopsy procedure. The specimens from patients with poorly healed ulcers as well as tissues from an equal number of randomly chosen patients with well-healed ulcers were processed for examination of TGF-β and TGF-β receptor II by RT-PCR and in situ hybridization.
Expression patterns of TGF-β and TGF-β receptor II were assayed by RT-PCR. Total RNA from specimens of gastric epithelium was prepared using Trizol reagent and converted to cDNA by using a reverse primer and reverse transcriptase (Invitrogen, Carlsbad, CA, USA). To amplify the cDNA, we used Taq DNA polymerase and performed PCR consisting of 40 cycles at 94 °C for 30 s, at 65 °C for 30 s, and at72 °C for 1 min. The specific TGF-β primer sequences used were: 5’-GCAGAACCCAAAAGCCAGAGTG -3’ and 3’-AGTTGGAGGTGCCATCAATACC -5’ (producing a 314 bp fragment). Specific TGF-β receptor II primer sequences were: 5’- ACCTGCTGCCTGTGTGACTTTG-3’ and 3’-TTTGGTAGTGTTTAGGGAGCCG-5’(producing a 528 bp fragment). Actin was used as a positive control, and the actin primer sequences were 5’-AACCATGAGGGAAATCGYGCAC-3’ and 3’-AGTCAAGGGAATCGGCAGAATG-5’ (producing a 419 bp fragment).
For in situ hybridization, specimens were initially fixed in 4% paraformaldehyde in 0.1 mol/L phosphate buffer and were then embedded in paraffin. The sections were subsequently deparaffinized and rinsed three times in phosphate-buffered saline (PBS), acetylated with acetic anhydride, washed again in PBS, and then placed in prehybridization solution. This solution was replaced with hybridization solution containing a digoxigenin (DIG)-labeled cRNA probe. cRNA probes prepared from selected TGF-β and TGF-β receptor II cDNA were incubated with the sections in a humidified chamber at 60 °C overnight. Stringency washes were performed at 60 °C in 0.2×SSC (sodium chloride/ sodium citrate) for 2 h after hybridization. Finally, the sections were blocked with 10% normal goat serum for 1 h, incubated with anti-DIG antibody (1:500; Roche, Mannheim, Germany) overnight at 4 °C, and reacted with a NBT/BCIP detection solution. Sections were air-dried and mounted with crystal mount (Biomeda, Foster City, CA, USA), and then photographed under an Axioskop brightfield microscope (Carl Zeiss, Oberkochen, Germany).
We obtained polyclonal TGF-β and TGF-β receptor II antibodies from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and the secondary antibody, biotinylated goat anti-rabbit immunoglobulin from Sigma (Sigma, St. Louis, MO, USA).
Sections were rinsed in PBS, incubated in 3% hydrogen peroxide solution for 30 min to eliminate endogenous peroxidase activity, and finally blocked for 1 h in PBS containing 5% goat serum and 0.5% Triton X-100. All primary antibodies were applied for 2 h at room temperature and then rinsed three times for 5 min with PBS. The secondary antibody, biotinylated goat anti-rabbit immunoglobulin (Vector Labs., Burlington, Canada), were applied at a 1: 200 dilution in PBS for 1 h. Labeling was accomplished with a Vector ABC kit (Vector Labs., Burlington, Canada) in diaminobenzidine (DAB) reaction (Sigma, St. Louis, MO, USA).
Eighty-five patients were considered for enrollment. Eleven did not complete follow up. Of the 74 patients who completed the entire treatment and follow up, 8 had a poor response to anti-ulcer treatment. Therefore, 8 of the 66 patients with well-healed ulcers were randomly selected as controls. Of the 16 study patients, 10 were males and 6 females. The average age was 52.5 years. The patients’ characteristics, a description of their ulcers before and after treatment, and the medication given are shown in Table 1.
| No. | Age (yr) | Sex | Location | Shape | Stage2 | (cm) | Stage3 | (cm) | HP | Treatment |
| 1 | 73 | M | Antrum | Round | A1 | 1 | - | + | Ranitidine | |
| 2 | 35 | F | Angle | Triangle | A1 | 0.8 | S2 | + | Nizatidine | |
| 3 | 42 | M | Body | Ovoid | A2 | 0.9 | - | - | Ranitidine | |
| 4 | 61 | M | Body | Ovoid | A2 | 1.2 | - | + | Ranitidine | |
| 51 | 58 | F | Angle | Round | A2 | 1.2 | H1 | 0.6 | - | Ranitidine |
| 61 | 42 | M | Antrum | Triangle | A1 | 0.8 | H2 | 0.5 | - | Nizatidine |
| 71 | 51 | M | Cardia | Round | A2 | 1.3 | H1 | 0.7 | + | Ranitidine |
| 81 | 68 | M | Body | Ovoid | A1 | 0.9 | H2 | 0.5 | + | Nizatidine |
| 9 | 54 | M | Angle | Round | A2 | 1 | S2 | + | Ranitidine | |
| 10 | 62 | F | Cardia | Round | A1 | 1.2 | - | - | Nizatidine | |
| 11 | 39 | F | Antrum | Ovoid | H1 | 0.8 | - | + | Nizatidine | |
| 12 | 71 | M | Angle | Round | A2 | 1 | S2 | - | Ranitidine | |
| 131 | 62 | F | Antrum | Triangle | A1 | 1.2 | H2 | 0.6 | + | Nizatidine |
| 141 | 36 | M | Body | Ovoid | H1 | 0.7 | H2 | 0.4 | - | Ranitidine |
| 151 | 46 | F | Angle | Round | A1 | 1.3 | H1 | 0.9 | + | Ranitidine |
| 161 | 40 | M | Antrum | Ovoid | A2 | 0.9 | H2 | 0.6 | + | Nizatidine |
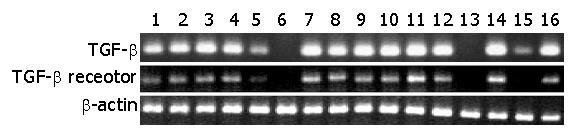
Figure 1 shows the results of PCR analysis of mRNA expression. TGF-β and TGF-β receptor II mRNA were easily detected in the gastric mucosa of the 8 patients with good ulcer healing. Among the 8 patients with poor ulcer healing, 4 had findings similar to those with well-healed ulcers. However, in the remaining 4 patients, mRNA expression was reduced or absent. Patient 5 had reduced expression of mRNA for both TGF-β and TGF-β receptor II, patient 15 had reduced TGF-β mRNA and absent TGF-β receptor II mRNA, while no mRNA for either was detected in patients 6 and 13.
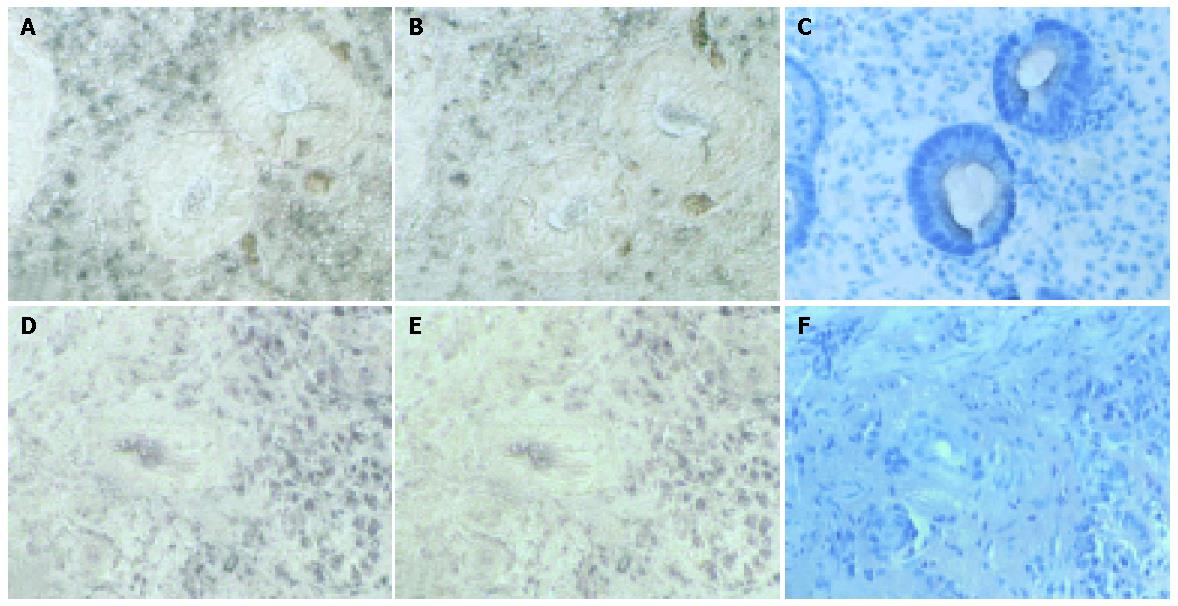
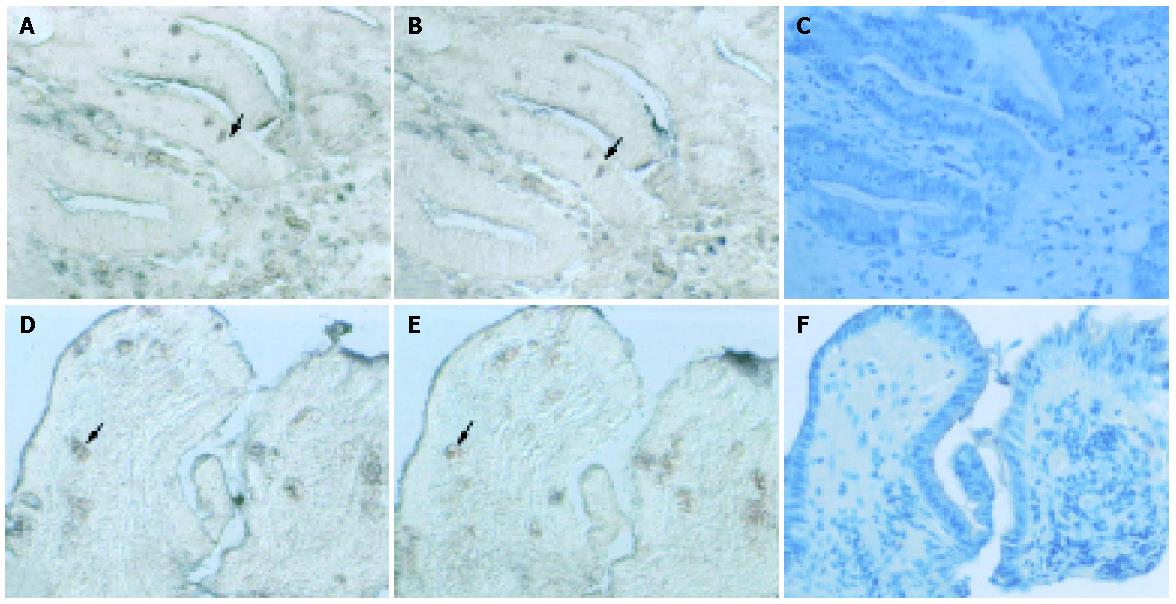
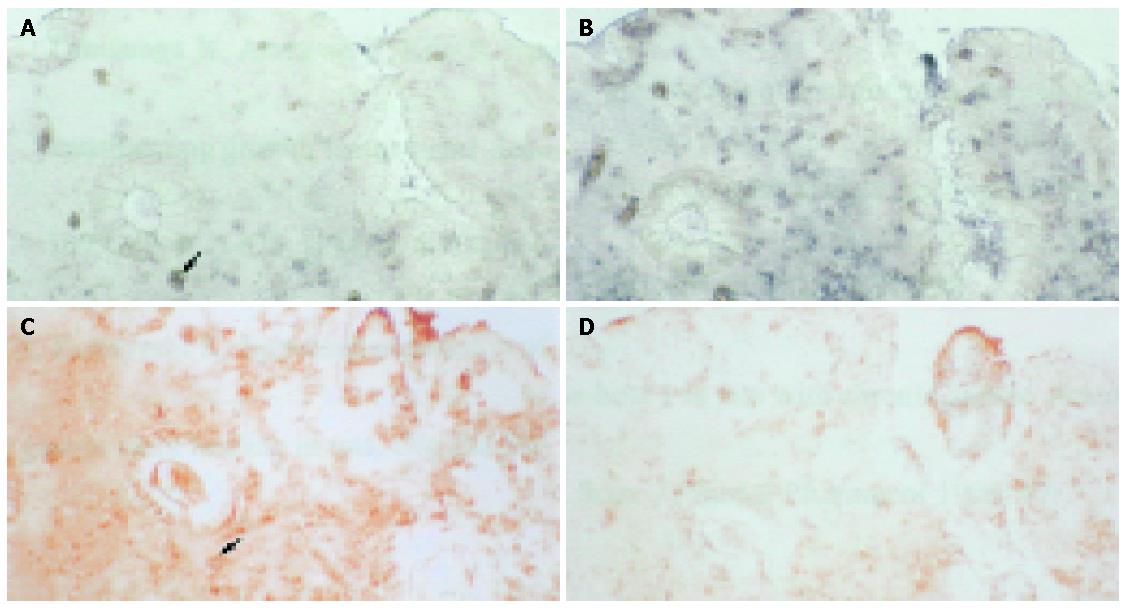
Examples of expression patterns based on in situ hybridization are illustrated in Figures 2-4. In patients 1 and 2, who had well-healed ulcers, TGF-β and TGF-β receptor II transcripts were detected not only in the epithelial cells but also in the cells of the lamina propria, where they were more highly expressed than in the epithelium (Figure 2). In patients 3 and 4, also with well-healed ulcers, TGF-β transcripts were present in the cells of the lamina propria as well as epithelial cells, with TGF-β receptor II mRNA detected in the same area (Figure 3, arrowheads). In patient 5, who had poor ulcer healing, of only a small amount TGF-β mRNA was seen in the gastric epithelium and in the lamina propria, with minimal TGF-β receptor II mRNA expression detectable in the same region (Figure 4A,B, arrowheads). In patient 6, very few TGF-β mRNA signals were detected, and there was no evidence of TGF-β receptor II expression (Figures 4D, E).
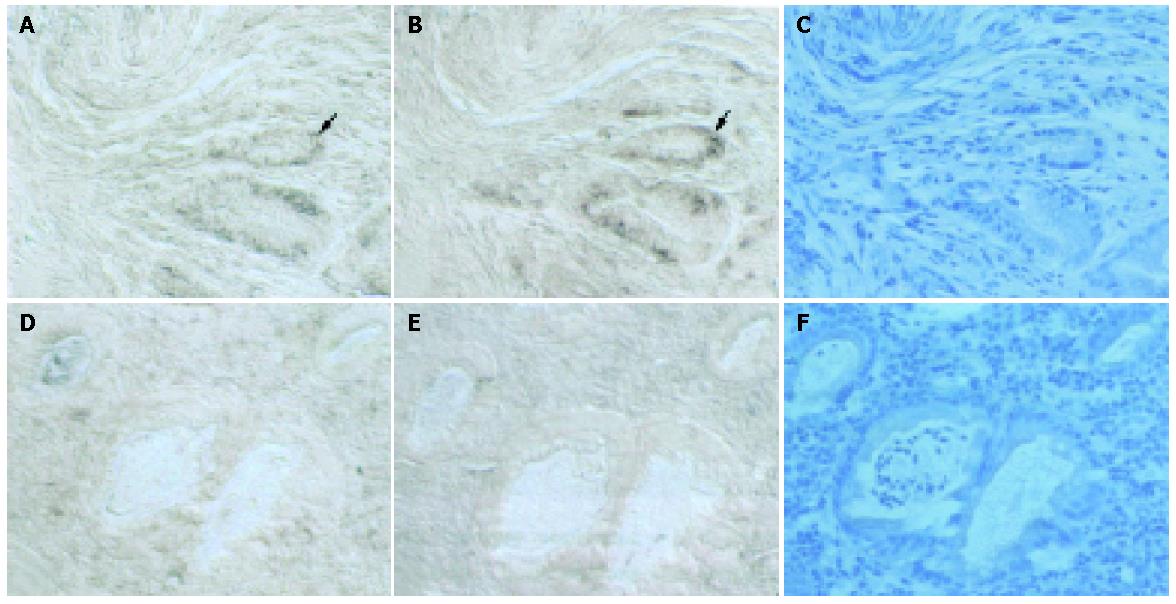
Results of in situ hybridization and immunohistochemistry to localize TGF-β and TGF-β receptor II in the gastric mucosa of patient 7 with good ulcer healing are shown in Figure 5. TGF-β protein and mRNA were present in the same areas (Figure 5A,C, arrowheads). TGF-β receptor II mRNA was detected in the gastric epithelium and in the lamina propria, where the TGF-β receptor II protein was also detected (Figures 5B,D). Immunohistochemistry and in situ hybridization thus confirmed the results of RT-PCR.
Ulcers that fail to heal after 12 weeks’ treatment are called refractory or intractable ulcers. Both gastric and duodenal ulcers are included in the category of peptic ulcer diseases, but the pathogenesis differs. In duodenal ulcers, hyperacidity and Helicobacter pylori infection are two critical contributing factors[6,7]. Therefore, ulcers in the duodenum refractory to treatment with standard doses of antacids or H2-blockers often respond to more potent acid suppression with proton pump inhibitors[8]. Acid secretion and H pylori are not critical in the development of gastric ulcers. Instead, factors such as abnormalities of motility[9,10] and distribution of mucosal blood flow have been implicated. Abundant muscle bundles combined with relative hypoperfusion may explain why refractory ulcers are particularly common in the gastric angle, according to some reports[11].
Since acid secretion is not essential for the development of gastric ulcers[12], there have been few reports concerning proton pump inhibitors for treatment of refractory ulcers[13]. In our previous study[5], we used H2 blockers. In order to make a fair comparison, we also used H2 blockers in this study, despite the increasing popularity of proton pump inhibitors.
Factors reported to be associated with poor healing of gastric ulcers include poor compliance with therapy, smoking, tolerance to H2 blockers, persistence of H pylori infection, continued use of NSAIDs and malignancy or other unusual causes of ulceration having been mistaken for benign ulcers[14-17]. Despite exclusion of all those factors, however, some patients still have inadequate ulcer healing after appropriate treatment of adequate duration. Twelve of 59 patients in our previous study[5] and 8 of 74 patients in the current study had a poor response to treatment. Of the 20 patients with refractory gastric ulcer, 11 had H pylori and six had ulcers in the gastric angle. Based on these observations, we are not convinced that either H pylori infection or location of the ulcer plays a determining role in the development of ulcer intractability. Therefore, other than the causes usually mentioned in the literature, it appears that locally acting trophic factors also contribute substantially to the healing of gastric ulcers[18-20].
In our previous immunohistochemical study, we have provided direct evidence that TGF-β is expressed in patients during gastric ulcer healing, and that insufficiency of TGF-β or its receptors may be associated with poor healing[5]. In the current study, we extended these observations using RT-PCR and in situ hybridization to demonstrate lower or absent TGF-β and TGF-β receptor II mRNA expression in poorly healed gastric ulcers as compared with the gastric mucosa near well-healed ulcers. RT-PCR is an efficient method extensively used in many fields for diagnosis. Based on this study, we suggest that RT-PCR analysis of TGF-β and its receptors in gastric samples may be worth further investigation, both to elucidate the healing process and predict good or poor healing. The latter requires testing samples both before and after treatment.
The healing process of wounds or ulcers involve several different trophic factors, and TGF-β is not the only one[18-20]. This may explain why some patients with refractory gastric ulcer still have some expression of TGF-β and its receptors. Nevertheless, the contrast with the abundant expression in all patients with well-healed ulcers reinforces previous findings implying that TGF-β plays an important role in the healing of gastric ulcers[1,2,5,21].
As TGF-β receptors I and II cooperate for TGF-β signal transduction, they are both apparently required in a particular cell to ensure TGF-β function. TGF-β receptor II is a serine/threonine kinase first activated by the TGF-β ligand. Subsequently, TGF-β receptor I is recruited and phosphorylated by TGF-β receptor II to form an active receptor complex[2,22]. In this study by in situ hybridization, we observed that cells expressing TGF-β and TGF-β receptor II could be accurately localized in the mucosal tissue. Interestingly, some epithelial cells expressed both TGF-β and TGF-β receptor II (Figures 3 and 4), suggesting that TGF-β and TGF-β receptor II may be auto-regulated in these cells.
In conclusion, our study confirms the absence or reduction of TGF-β and TGF-β receptor II mRNA in the gastric mucosa of some patients with refractory gastric ulcers. The mRNA of TGF-β and TGF-β receptor II as well as the proteins themselves could be colocalized in the gastric mucosa of patients with good ulcer healing by the combined use of immunohistochemistry and in situ hybridization.
Facilities provided by the Program for Promoting Academic Excellence of Universities (89-B-FA01-1-4) of the Ministry of Education are also gratefully acknowledged.
Edited by Wang XL and Zhu LH
| 1. | Tominaga K, Arakawa T, Kim S, Iwao H, Kobayashi K. Increased expression of transforming growth factor-beta1 during gastric ulcer healing in rats. Dig Dis Sci. 1997;42:616-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Ernst H, Konturek PC, Brzozowski T, Konturek SJ, Hahn EG. Subserosal application of transforming growth factor-beta 1 in rats with chronic gastric ulcers: effect on gastric ulcer healing and blood flow. J Physiol Pharmacol. 1996;47:443-454. [PubMed] |
| 3. | Massagué J. Receptors for the TGF-beta family. Cell. 1992;69:1067-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 491] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 4. | Kingsley DM. The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 1994;8:133-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 1384] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 5. | Shih SC, Chien CL, Tseng KW, Lin SC, Kao CR. Immunohistochemical studies of transforming growth factor-beta and its receptors in the gastric mucosa of patients with refractory gastric ulcer. J Formos Med Assoc. 1999;98:613-620. [PubMed] |
| 6. | Lam SK. Pathogenesis and pathophysiology of duodenal ulcer. Clin Gastroenterol. 1984;13:447-472. [PubMed] |
| 7. | Olbe L, Fandriks L, Hamlet A, Svennerholm AM, Thoreson AC. Mechanisms involved in Helicobacter pylori induced duodenal ulcer disease: an overview. World J Gastroenterol. 2000;6:619-623. [PubMed] |
| 8. | Bardhan KD. Omeprazole in the management of refractory duodenal ulcer. Scand J Gastroenterol Suppl. 1989;166:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Fisher R, Cohen S. Physiological characteristics of the human pyloric sphincter. Gastroenterology. 1973;64:67-75. [PubMed] |
| 10. | Fisher RS, Cohen S. Pyloric-sphincter dysfunction in patients with gastric ulcer. N Engl J Med. 1973;288:273-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Valle JD, Chey WD, Scheiman JM. Acid Peptic Disorders. In; Yamada T, Alpers DH, Kaplowitz N, Laine L, Owyang C, Powell DW. Textbook of Gastroenterology. 4th edition, Philadelphia: Lippincott Williams Wilkins 2003; 1322-1328. |
| 12. | Reid J, Taylor TV, Holt S, Heading RC. Benign gastric ulceration in pernicious anemia. Dig Dis Sci. 1980;25:148-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Brunner G, Arnold R, Hennig U, Fuchs W. An open trial of long-term therapy with lansoprazole in patients with peptic ulceration resistant to extended high-dose ranitidine treatment. Aliment Pharmacol Ther. 1993;7 Suppl 1:51-55, discussion 61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Nwokolo CU, Smith JT, Gavey C, Sawyerr A, Pounder RE. Tolerance during 29 days of conventional dosing with cimetidine, nizatidine, famotidine or ranitidine. Aliment Pharmacol Ther. 1990;4 Suppl 1:29-45. [PubMed] |
| 15. | Wilder-Smith C, Halter F, Ernst T, Gennoni M, Zeyen B, Varga L, Roehmel JJ, Merki HS. Loss of acid suppression during dosing with H2-receptor antagonists. Aliment Pharmacol Ther. 1990;4 Suppl 1:15-27. [PubMed] |
| 16. | Podolsky I, Storms PR, Richardson CT, Peterson WL, Fordtran JS. Gastric adenocarcinoma masquerading endoscopically as benign gastric ulcer. A five-year experience. Dig Dis Sci. 1988;33:1057-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Lanas AI, Remacha B, Esteva F, Sáinz R. Risk factors associated with refractory peptic ulcers. Gastroenterology. 1995;109:1124-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Chen MC, Lee AT, Soll AH. Mitogenic response of canine fundic epithelial cells in short-term culture to transforming growth factor alpha and insulinlike growth factor I. J Clin Invest. 1991;87:1716-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Wright NA, Pike CM, Elia G. Ulceration induces a novel epidermal growth factor-secreting cell lineage in human gastrointestinal mucosa. Digestion. 1990;46 Suppl 2:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Milani S, Calabrò A. Role of growth factors and their receptors in gastric ulcer healing. Microsc Res Tech. 2001;53:360-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Coerper S, Sigloch E, Cox D, Starlinger M, Köveker G, Becker HD. Recombinant human transforming growth factor beta 3 accelerates gastric ulcer healing in rats. Scand J Gastroenterol. 1997;32:985-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Wrana JL, Attisano L, Wieser R, Ventura F, Massagué J. Mechanism of activation of the TGF-beta receptor. Nature. 1994;370:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1743] [Cited by in RCA: 1766] [Article Influence: 57.0] [Reference Citation Analysis (0)] |