Published online May 1, 2004. doi: 10.3748/wjg.v10.i9.1379
Revised: September 20, 2002
Accepted: November 4, 2002
Published online: May 1, 2004
AIM: To study the role of hybrid bioartificial liver (HBL) in clearing proinflammatory cytokines and endotoxin in patients with acute and sub-acute liver failure and the effects of HBL on systemic inflammatory syndrome (SIRS) and multiple organ dysfunction syndrome (MODS).
METHODS: Five cases with severe liver failure (3 acute and 2 subacute) were treated with HBL. The clinical signs and symptoms, total bilirubin (TBIL), serum ammonia, endotoxin TNF-α, IL-6 and prothrombin activity (PTA), cholinesterase (CHE) were recorded before, during and after treatment. The end-stage liver disease (MELD) was used for the study.
RESULTS: Two patients were bridged for spontaneous recovery and 1 patient was bridged for OLT successfully. Another 2 patients died on d 8 and d 21. The spontaneous recovery rate was 30.0%. PTA and CHE in all patients were significantly increased (P < 0.01), while the serum TBIL, endotoxin,TNF-α, IL-6 were decreased. MELD score (mean 43.6) predicted 100% deaths within 3 mo before treatment with HBL. After treatment with HBL, four out of 5 patients had decreased MELD scores (mean 36.6). The MELD score predicted 66% mortalities.
CONCLUSION: The proinflammatory cytokines (TNFα, IL-6 and endotoxin)can be significantly removed by hybrid bioartificial liver and HBL appears to be effective in blocking SIRS and MODS in patients with acute and sub-acute liver failure. MELD is a reliable measure for predicting short-term mortality risk in patients with end-stage liver disease. The prognostic result also corresponds to clinical outcome.
- Citation: Liu Q, Duan ZP, Huang C, Zhao CH. Evaluation of effect of hybrid bioartificial liver using end-stage liver disease model. World J Gastroenterol 2004; 10(9): 1379-1381
- URL: https://www.wjgnet.com/1007-9327/full/v10/i9/1379.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i9.1379
About 80% ICU deaths may be attributable to progressive multiple organ dysfunction (MODS). MODS is also a major cause of death in patients suffering from severe hepatitis. The sequela of systemic inflammatory syndrome (SIRS) is MODS. Hybrid bioartificial liver (HBL) is a well-known liver support system that is close to native liver in function. In the present study our primary goals were to investigate the role of HBL in removing serum proinflammatory cytokines such as TNFa, IL-6, endotoxin and in blocking SIRS to MODS.
In this report, we used the validity of end-stage liver disease model as a disease severity index for patients with end-stage liver disease treated with bioartificial liver support system. The model was considered to be able to provide a reliable estimate of short-term survival over a wide range of liver diseases.
The Beijing Youan Hospital Ethics Committee approved the study and informed consent was obtained from all patients or their relatives. Five patients with severe hepatitis treated with HBL in our center from March, 2000 to June 2001 were involved in the study. Three of 5 patients were acute severe hepatitis and 2 of them were sub-acute hepatitis. All the 5 patients suffered from SIRS, 4 of them were complicated with 2-organ failure and only one patient were with 3-organ failure (Table 1). The criteria for severe hepatitis, SIRS and MODS were based on the recommendations of references 1-3[1-3].
| No | Sex | Age(yr) | Diagnosis | Multi-organs failure | Outcome |
| 1 | Female | 43 | ALF, HBV | Liver, brain | Spontaneous recovery |
| 2 | Male | 31 | SALF, HBV | Liver, brain | Recovery spontaneous |
| 3 | Male | 33 | SALF, HBV and alcoholic liver disease | Liver, coagulopathy, cardiovascular | Orthotopic liver transplantation |
| 4 | Female | 27 | ALF, drug induced liver injury | Liver, brain | Death |
| 5 | Male | 31 | ALF, HBV | Liver, kidney | Death |
HBL system consists of a bioreactor (Micrognlnc, Laguna Hills, CA) with a pore size 0.2 μm, total fiber internal surface area 6000 cm2, external surface area 7000 cm2, volume 200 mL loaded with fresh isolated hepatocytes (2 × 1010-4 × 1010), a single-use plasma circuit,and a machine to control the fluid flow through these components. At first, plasma exchange was achieved by a membrane separation method (PLASAUTO-IQ, Asahi, Tokyo). The amount of plasma removal was set at 3000 mL. In the following HBL treatment (SYBIOL, United States), patients’ plasma was separated using a plasma separation system (COBE-Spectra,Lakewood,CO) and was then perfused through an HBL system. The plasma was separated at a rate of 70-100 mL/min and perfused through the bioreactor at a rate of 28-30 mL/min; flow rate of porcine cell suspension was 60 mL/min. After exchange of patients’ plasma with porcine cells across the hollow-fiber membranes, patients’ blood was returned to the superficial femoral vein. Evaluation of the viability of hepatocytes in circuit of porcine liver cells was done by cell membrane exclusion of trypan blue dye.
All patients were managed in a specialized intensive care unit and received standard supportive care. The following parameters were monitored before, during and after treatment: vital signs (blood pressure, heart rate, breath rate and SaO2), hemodynamics (mean artery pressure, central venous pressure) and urinary output. Liver biochemistry [bilirubin, transaminases, Creatinine, BUN, serum ammonia, prothrombin activity (PTA)], cytokines TNTa, IL-6 (ELISA, Bangding Co) and endotoxin were analysed. The mental state of hepatic encephalopathy was graded into five stages. When the patients regained consciousness or hepatic encephalopathy decreased by I stage, the treatments was considered as effective.
According to Kamath et al, the score was multiplied by 10 and then rounded to the nearest integer. The formula for the MELD scores 3.8 × loge[bilirubin (mg/dL)] + 11.2 × loge(INR) + 9.6 × loge [creatinine (mg/dL)]. We used on-line available worksheet to compute MELD scores (http://www.mayo.edu/int-med/gi/model/ mayomodl.htm).
Statistical comparisons were carried out using chi-square and Student t-tests. A P value < 0.05 was considered statistically significant.
All treatments were completed as scheduled. Each treatment lasted for 8-10 h and all were well tolerated. All patients remained hemodynamically stable throughout the treatment period. No gastrointestinal coagulopathy, fever, allergy and other severe adverse reactions were observed.
Gastrointestinal symptoms such as anorexia, nausea and fatigue were sighificantly improved in 4 out of 5 patients Three patients who suffered from hepatic encephalopathy (HE) experienced remarkable neurological improvement with reversal of the decerebrate state after HBL treatment. Two of 3 HE at grades III and II became respectively grades II and I, another one at grade IV regained consciousness. Four patients who were complicated with 2-organ failure recovered spontaneously, one died 8 and 21 d after HBL treatment. The patient who was complicated with 3-organ failure (liver, cardiovascular, and gastrointestinal coagulopathy) was successfully bridged until an organ became available for orthotopic liver transplantation 1 wk after HBL treatment. The spontaneous recovery rate was 40.0%.
The treatment with the PE lowered TBIL in all patients (from 23.08 ± 12.50 mg/dL to 12.34 ± 4.39 mg/dL). At the end of treatment TBIL was reduced about 50%. Post evaluation showed that TBIL had a significant difference before and after HBL treatment at 0, 24 and 72 h (P < 0.05). No statistically significant improvement in albumin and blood ammonia levels noted (P < 0.05). CHE and PTA were significantly increased in all patients at 0, 24, 72 h and at the end of HBL treatment (P < 0.05-0.01). The serum chemistry changes are shown in Table 2.
| Item | TBIL (mg/dL) | NH3 (µg/dL) | ALB (g/L) | CHE (U/L) | PTA% |
| Pre-treatment | 23.08 ± 12.50 | 116.6 ± 23.1 | 29.79 ± 4.35 | 3801.5 ± 1710.6 | 17.25 ± 10.11 |
| Post-treatment at 0 h | 12.34 ± 4.39b | 98.3 ± 19.5 | 32.57 ± 4.76 | 5560.9 ± 1067.6a | 38.60 ± 10.25b |
| Post-treatment at 24 h | 14.10 ± 6.08b | 103.2 ± 23.5 | 33.13 ± 4.34 | 5438.5 ± 1024.3a | 34.31 ± 11.75a |
| Post-treatment at 72 h | 17.24 ± 8.34a | 110.8 ± 30.6 | 31.42 ± 4.07 | 5676.2 ± 957.9a | 35.20 ± 9.78b |
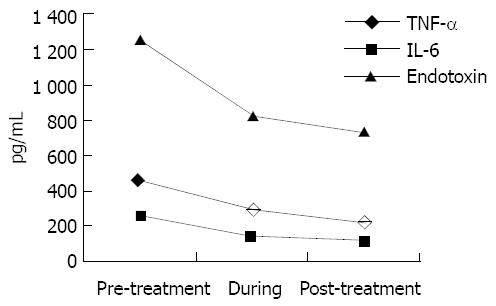
A significant decrease in serum endotoxin levels was observed by the end of PE (P < 0.05) and it was declined during the period of HBL treatment. By the end of HBL, endotoxin level was the lowest (P < 0.001). TNF-α and IL-6 were reduced rapidly, the clearance rate of TNF-α was approximately 45% and IL-6 was 55% (P < 0.001) (Figure 1).
MELD was used for predicting the outcome of HBL before and after treatment (Table 3).
| Patients | Before-treatment | After-treatment | ||||||||||
| 1 | 2 | 3 | 4 | 5 | mean ± SD | 1 | 2 | 3 | 4 | 5 | mean ± SD | |
| PTA% | 22.4 | 21 | 18 | 11 | 14.37 | 17.35 ± 4.7 | 40.2 | 43 | 45 | 30.1 | 18.2 | 35.3 ± 20.87 |
| INR | 2.42 | 2.51 | 2.61 | 4.14 | 3.74 | 3.08 ± 1.27 | 1.58 | 1.55 | 1.5 | 2.08 | 2.71 | 1.8 ± 1.02 |
| Creatinine (mg/dL) | 13 | 16.9 | 7.9 | 16.6 | 42.4 | 19.36 ± 15.3 | 5.9 | 5 | 4.3 | 8.9 | 35.2 | 11.8 ± 13.1 |
| Bilirubin (mg/dL) | 20.4 | 17 | 15.1 | 22.2 | 40.46 | 23.03 ± 10.1 | 20.4 | 17 | 15.1 | 22.2 | 40.46 | 17.4 ± 12.66 |
| MELD | 41 | 41 | 41 | 47 | 48 | 43.6 | 34 | 33 | 32 | 39 | 45 | 33.6 |
In the present study we demonstrated that the patients with severe hepatitis and hepatic failure might benefit from HBL for temporary liver support. HBL could maintain patients with hepatic failure alive, neurologically intact and metabolic state improved until a donor organ was available[4]. It could also allow the native liver to regenerate, thus avoiding liver transplantation. The study showed that TNF-α and IL-6 were reduced rapidly about 45% and 55% (P < 0.001) after HBL treatment. The alteration of serum endotoxin levels was similar to TNFa and IL-6. Four of 5 patients responded to HBL treatment and 3 of them survived. Since the case number was limited, there were not enough data in our study to show that HBL could improve the survival rate of patients with multiple organ dysfunction. However the beneficial effect of HBL on the systemic concentration of cytokines was definite and the efficacy was not reached by drug treatment in such a short period. Just as Stephen described[5,6], a success liver support device was likely to depend on their ability to modify factors influencing liver cell death and regeneration and on the extent to which multi-organ failure could be prevented or reversed. Hybrid liver support devices replace the lost liver function through a combination of mechanical and biologic effects Mechanical parts use activated charcoal or plasma exchange before the hepatocyte bioreactor, which may not only provide a “protective” effect on the porcine hepatocytes from possible toxic effect of hepatic failure plasma, but also strengthen the effect of plasma detoxification. HBL treatment could remarkably improve SIRS signs and symptoms. Also it slowed down the progression from SIRS to MODS and protected MODS from further deterioration.
The mechanism of HBL to block the progression from SIRS to MDOS was to support failed liver. In our study, It was observed that CHE and PTA were significantly increased in all patients at 0, 24 and 72 h after HBL treatment (P<0.05-0.01). Plasma cholinesterase, an enzyme with a short half-life, is not usually changed by the substitution of clotting factors or by freshly frozen plasma and therefore is a good parameter of synthesis. Increased cholinesterase level in plasma may reflect improvement of liver synthesis function. The metabolic ability of liver for various nervous toxicity implicates liver detoxification function. Similar to the previous report, 3 patients with hepatic encephalopathy were improved by HBL treatment in our study, but it was different from previous report, in that the blood ammonia levels were not changed after HBL treatment. Impairment of central nervous system in hepatic encephalopathy is probably multifactorial in origin. Except ammonia, other neuroexcitatory factors like amino acid imbalance, mercaptans, phenols, fatty acids, GABA, and benzodiazepine, also play a role in hepatic encephalopathy. Therefore consciousness improvement may cause removal of other neuroexcitatory factors. There are two mechanisms blocking the progression from SIRS to MODS related to liver function improvement. First, liver is an amplifier of the systemic acute-phase reaction, proinflammatory cytokines enter the liver through the portal vein, eventually passing the smallest functional unit of the liver, the acnus. The special architecture of sinusoids ensures that cytokines in the liver primarily reach the nonparenchymal liver cells such as endothelial cells and kupffer cells. Because these cells express not only receptors of IL-1β, TNF-α and IL6, but also modulators of cytokines[7]. Also the most active cells howe the potential for the systemic response to the intrahepatic release of inflammatory cytokines. They might function as intrahepatic amplifiers of the systemic acute-phase reaction by liberating a second wave of proinflammatory cytokines which could enhance the effect of injuring agents and accelerate or maintain development of liver fibrosis[8]. Second, it has been found that LBP/CD14 is an endotoxin strengthening system. LBP is produced in liver. When under stress the production of LBP from liver would increase many times. Trace endotoxin binding to LBP and interacting with target cells would increase the sensitivity of target cells to endotoxin up to10 000 times and stimulate the synthesis and secretion of a large number of proinflammatory cytokines, mainly IL1B and TNFa[8]. Therefore the improvement in liver function is the basis for decreasing proinflammatory cytokine levels.
MELD is a reliable measure and predictive of short-term mortality risk in patients with end-stage liver disease[9].
Edited by Wang XL, Zhu LH Proofread by Xu FM
| 1. | Fifth conference of national contagious disease and parasitic dis-ease Guideline: prevention and treatment of virus hepatitis (draft). Zhonghua Neike Zazhi. 1995;34:788-791. |
| 2. | Qiu HB, Zhou SX, Yang Y. Multiple organ dysfunction syndrom predictors of mortality and clinical therapetic strategies. Linchuang Jjijiuy Yixue. 2001;10:13-16. |
| 3. | Fry DE, Pearlstein L, Fulton RL, Polk HC. Multiple system organ failure. The role of uncontrolled infection. Arch Surg. 1980;115:136-140. |
| 4. | Xue YL, Zhao SF, Zhang ZY, Wang YF, Li XJ, Huang XQ, Luo Y, Huang YC, Liu CG. Effects of a bioartificial liver support system on acetaminophen induced acute liver failure canines. World J Gastroenterol. 1999;5:308-311. |
| 5. | Zimmerman JE, Knaus WA, Sun X, Wagner DP. Severity stratification and outcome prediction for multisystem organ failure and dysfunction. World J Surg. 1996;20:401-405. |
| 6. | Riordan SM, Williams R. Acute liver failure: targeted artificial and hepatocyte-based support of liver regeneration and reversal of multiorgan failure. J Hepatol. 2000;32:63-76. |
| 7. | Ramadori G, Christ B. Cytokines and the hepatic acute-phase response. Semin Liver Dis. 1999;19:141-155. |
| 8. | Stange J, Mitzner SR, Risler T, Erley CM, Lauchart W, Goehl H, Klammt S, Peszynski P, Freytag J, Hickstein H. Molecular adsorbent recycling system (MARS): clinical results of a new membrane-based blood purification system for bioartificial liver support. Artif Organs. 1999;23:319-330. |
| 9. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. |