Published online May 1, 2004. doi: 10.3748/wjg.v10.i9.1310
Revised: October 10, 2003
Accepted: October 22, 2003
Published online: May 1, 2004
AIM: To investigate the significance of c-kit gene mutation in gastrointestinal stromal tumors (GIST).
METHODS: Fifty two cases of GIST and 28 cases of other tumors were examined. DNA samples were extracted from paraffin sections and fresh blocks. Exons 11, 9 and 13 of the c-kit gene were amplified by PCR and sequenced.
RESULTS: Mutations of exon 11 were found in 14 of 25 malignant GISTs (56%), mutations of exon 11 of the c-kit gene were revealed in 2 of 19 borderline GISTs (10.5%), and no mutation was found in benign tumors. The mutation rate showed significant difference (χ2 = 14.39, P < 0.01) between malignant and benign GISTs. Most of mutations consisted of the in-frame deletion or replication from 3 to 48 bp in heterozygous and homozygous fashions, None of the mutations disrupted the downstream reading frame of the gene. Point mutations and frame deletions were most frequently observed at codons 550-560, but duplications were most concentrated at codons 570-585. No mutations of exons 9 and 13 were revealed in GISTs, Neither c-kit gene expression nor gene mutations were found in 3 leiomyomas, 8 leiomyosarcomas, 2 schwannomas, 2 malignant peripheral nerve sheath tumors, 2 intra-abdominal fibromatoses, 2 malignant fibrous histiocytomas and 9 adenocarcinomas.
CONCLUSION: C-kit gene mutations occur preferentially in malignant GISTs and might be a clinically useful adjunct marker in the evaluation of GISTs and can help to differentiate GISTs from other mesenchymal tumors of gastrointestinal tract, such as smooth muscle tumors, schwannomas, etc.
- Citation: Hou YY, Tan YS, Sun MH, Wei YK, Xu JF, Lu SH, A-Ke-Su SJ, Zhou YN, Gao F, Zheng AH, Zhang TM, Hou WZ, Wang J, Du X, Zhu XZ. C-kit gene mutation in human gastrointestinal stromal tumors. World J Gastroenterol 2004; 10(9): 1310-1314
- URL: https://www.wjgnet.com/1007-9327/full/v10/i9/1310.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i9.1310
GISTs are the most common mesenchymal tumors of the human gastrointestinal tract, previously uniformly classified as smooth muscle tumors. Recently, GISTs have been defined as cellular spindle cell, epithelioid, or occasionally pleomorphic mesenchymal tumors of the gastrointestinal (GI) tract that express the c-kit protein (CD117), as detected using immunohist-ochemistry. GISTs are strongly and nearly uniformly CD117 positive[1-4].
Resently, the c-kit mutations in GISTs have been shown to lead to ligand-independent activation of the tyrosine kinase of c-kit[5]. So several questions were suggested: what are the possible relationship of c-kit expression and mutations, the relationship of mutations and malignancy, and the possible specificity of the mutations for GISTs?
A study of larger series of GISTs, revealed that these tumors had a spectrum of clinical behaviors at all sites of their occurrence[6]. Some GISTs are typical benign tumors, and most of them are found incidentally in other conditions, for example, during gall bladder or gynecologic surgery. In constrast, other GISTs metastasize to the liver and disseminate in the peritoneal cavity. Large tumor size, presence of intratumoral necrosis, infiltrative growth pattern in muscularis propria, invasion of mucosa, and high mitotic figures are considered as malignant. However, there is a definite percentage of mitotically inactive tumors that subsequently metastasize, emphasizing the fact that low mitotic count does not rule out a malignant behavior. Therefore, the designation “uncertain malignant potential” applies to a significant number of GISTs.
In this study, we further explored whether the c-kit gene mutations were valuable as an adjunct malignant marker in GISTs and could help to differentiate GISTs from other tumors.
Archival paraffin-embedded and frozen tissue samples of 52 GISTs were immunohistochemically analysed for CD117 and CD34. They were mainly obtained from the Department of Pathology, Zhongshan Hospital, Fudan University, and from other pathology departments in Cancer Hospital, Huadong Hospital, and Chongming Center Hospital, etc. Additionally, 28 other tumors were investigated, including 8 leiomyosarcomas of retroperitoneum, 3 leiomyomas of esophagus, 2 schwannomas of stomach, 2 malignant peripheral nerve sheath tumors of intra-abdomen, 2 intra-abdominal fibromatoses of intestine, 2 malignant fibrous histiocytomas of intra-abdomen, and 9 adenocarcinomas of GI (of which, 4 were accompanied by GISTs).
One to 14 hematoxylin and esosin-stained slides (median, 4 slides) were reviewed for each case. The following features were recorded in all cases: type of the majority cells (spindle vs epithelioid), skeinoid fibers (extracellular collagen globles), mucosal ulceration. Additionally, other important features included mitoses counted from 50 consecutive high-power fields (HPFs), coagulation necrosis, infiltrative growth pattern, and lymph node involvement. According to the histologic observation, GISTs were divided into 3 groups: small incidental tumors with less 5 cm in diameter or fewer mitoses per 50 HPF were designated as benign, mitotically inactive tumors larger than 5 cm or cellular tumors which had no necrosis and infiltration were designated as uncertain malignant potential, and tumors with active mitotic counts over five per 50 HPF or with necrosis, infiltrative growth pattern were designated as malignant.
Antibodies to the following antigens were used. CD117 (c-kit prooncogene product, polyclonal, 1:70) was purchased from Santa Cruz Biotechnology, Santa Cruz, CA, United States, CD34 (QBEND-10, monoclonal, 1:150, DAKO), α-SMA (1A4 monoclonal, 1:200), MSA (HHF35 monoclonal, 1:200), desmin (D33, monoclonal, 1:150), S-100 protein (polyclonal, 1:300), and protein gene product 9.5 (PGP9.5, polyclonal, 1:300) were purchased from Dako Corp. Epitope retrieval was used for antibodies. The slides were immersed in 10 mmol/L citrate buffer, pH 6.0 and heated in a microwave oven at a high setting for 5 min. The EnVision staining technique was used followed by incubation with 3,3’-diaminobenzidine tetrahydrochloride and counterstained with hematoxylin.
Ten micrometers of tissue sections were cut from freshy frozen tumors or paraffin embedded, and then incubated in extraction buffer (50 mmol/L KCl, 10 mmol/L Tris[pH8.3], 2.5 mmol/L MgCl2) containing 60 μg/mL proteinase K overnight at 55 °C. The proteinase K was inactivated by a 10-min incubation at 95 °C. The resulting lysate was spun in a microcentrifuge for 5 min to pellet debris, and then stored at -20 °C.
PCR assays were developed to amplify exons 11, 9, and 13. The 3 primer pairs were designed based on the human c-kit gene. Exons 9, 11 and 13 of the c-kit gene were amplified by PCR using the following oligonucleotide primer pairs: for exon 9, 5’-TCCTAGAGTAAGCCAGGGCTT-3’/5’-TGGTAGACAGAGC CTAAACATCC-3’; for exon 11, 5’-CCAGAGTGCTCTAATGA CTG-3’/5’-TGACATGG AAAGCCCCTGTT-3’; and for exon 13,5’-GCTTGACATCAGTTTGCCAG-3’/5’-AAAGGCAGCTT GGACACGGCTTTA-3’. The lengths of PCR products were 261 bp, 225 bp, and 193 bp, respectively. The PCR reaction conditions were the standard ones recommended by Perkin Elmer. The annealing temperature was 55 °C. The PCR products were size fractionated on 50 g/L polyacrylamide gels and stained with ethidium bromide.
Direct sequencing was performed on the ABI Prism 310 DNA sequencer, using the same primers as were used for amplification. Sequencing reactions were conducted with the big dye terminator sequencing ready reaction kit (Perkin-Elmer) according to the manufacturer’s instructions.
χ2 test was used.
There were 27 gastric, 7 small intestine, 6 rectal, 5 extra-GI, 2 abdominal disseminated primary tumors, with 5 intraabdominal recurrences. Of these tumors, 8 were classified as histologically benign, 19 as histologically uncertain malignant potential of borderline tumor, and 25 as malignant based on high mitotic activity, coagulative necrosis, infiltrative growth pattern. These tumors displayed a wide variety of cell types and histologic patterns, of which, 34 tumors had a predominant spindle cell pattern, 8 were epithelioid, and 10 were spindle/epithelioid mixed. The spindle cells were most often arranged in interlacing fascicles, The epithelioid cells were characterized by sheets. However, they often showed focally a storiform pattern, mimicked large rosettes, or palisaded the nuclei.
CD117 positivity was detected in 50 of 52 cases (96.2%), typically in most of tumor cells in a strong membrane of apparently diffuse cytoplasm. CD34 positivity was seen in 38 of 52 cases (73%), including focal reactive cases. Two CD117-negative cases were CD34 positive. A small number were focal positive for α-SMA (10 of 52), MSA (9 of 52), desmin (2 of 52). Additionally, 6 and 3 cases were reactive for S-100 and PGP9.5.
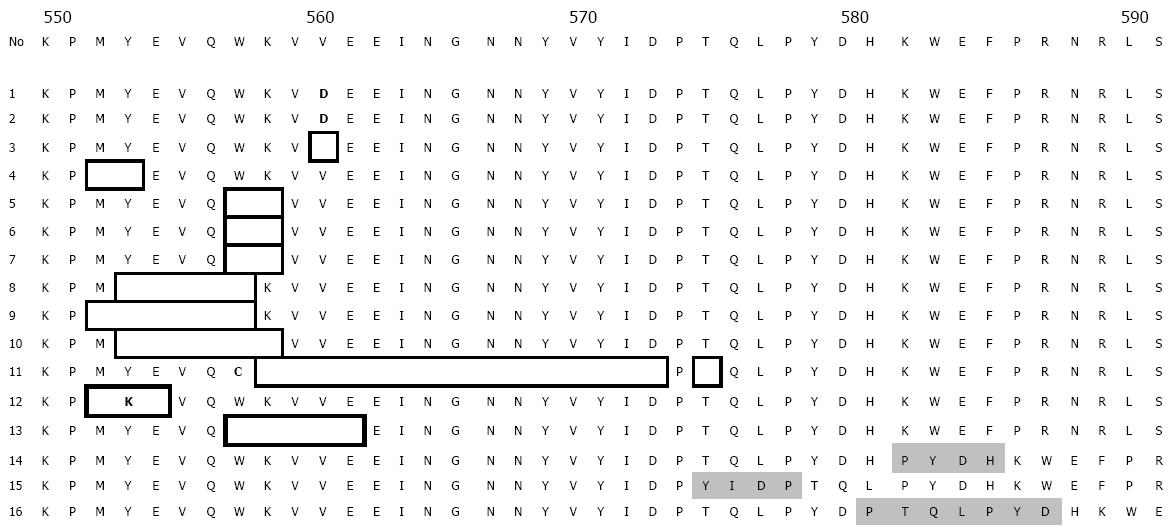
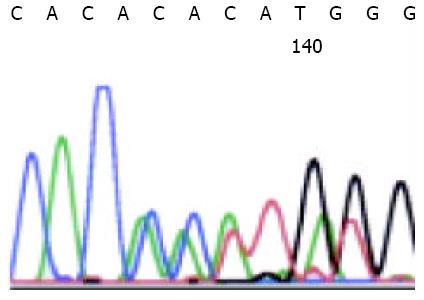
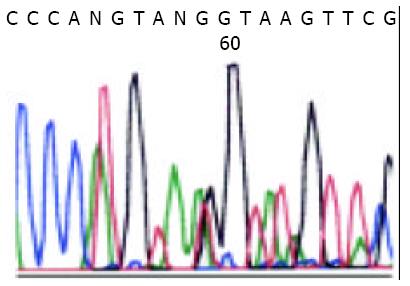
PCR amplification and DNA sequencing revealed exon 11 mutation in 14 malignant GISTs, in 2 borderline GISTs, and in no benign GISTs. Sequence analysis showed in-frame deletions of 3 to 48 bp, point mutations, and duplications. Point mutations and deletions were most frequently observed at codons 550-560, but duplications were most concentrated at codon 570-585. Of the 16 mutated GISTs, 2 had a homozygous mutation and 14 heterozygous mutation, which did not result in any amino acid change. The sequence corresponding to these mutations are shown in Figure 1. Figure 2 shows a case of duplication by DNA direct sequencing.
Five non-tumor tissue samples from the stomach or intestine adjacent to mutation-bearing GISTs demonstrated neither deletions nor point mutations.
The conservation of c-kit mutation pattern was observed in a relapse lesion from the same patients (Figure 3).
No exon 9 and exon 13 mutation were detected.
χ2 test showed that the c-kit mutation of GISTs demonstrated a statistical (χ2 = 14.39, P < 0.01) significance between the malignant group and the borderline and benign groups.
The three esophageal leiomyomas were histologically paucicellular spindle cell neoplasms with distinctly eosinophilic cytoplasm, and lack of mitotic activity. All cases were positive for α-SMA, MSA and desmin, and negative for CD117 and CD34, and only mast cells in the stroma showed positive CD117.
The 8 retroperitoneal leiomyosarcomas were spindle cell tumors that resembled smooth muscle tumor with oval to mildly elongated nuclei and variably eosinophilic cytoplasm. Four tumors showed focal pleomorphism and high mitotic activity (> 50/50HPF). Coagulation necrosis was present in 4 tumors. All cases were positive for α-SMA, MSA, and 7 cases positive for desmin. All cases were negative for CD34 but 1 case was focal weak positive for CD117.
The 2 cases of schwannomas showed slender spindle cells and a characteristic lymphoid cuff. Two cases were diffusely and strongly positive for S-100 and 1 case was positive for PGP9.5. Both of them were negative for CD117 and CD34. The 2 cases malignant peripheral nerve sheath tumor showed spindle cells and active mitosis. Two cases were positive for S-100 and weak positive for PGP9.5 and negative for CD117 and CD34.
The 2 cases of intro-abdominal fibromatosis of intestine and 2 cases of malignant fibrous histiocytoma were negative for all antigens but 1 case of intro-abdominal fibromatosis was focal positive for α-SMA.
There were no mutations of the c-kit gene in the above 28 control tumors and one case of adenocarcinoma accompanied by mutation-bearing GIST also had wild-type c-kit gene.
Most of the GISTs examined expressed both CD117 and CD34, which was consistent with the literature reports[7-12]. The rates of CD117 and CD34 expression had no difference between benign, borderline, and malignant GISTs, so the expressions were not useful markers for evaluating malignancy.
In this study, we examined the sequence and expression of c-kit gene in the spectrum of GISTs, including benign, borderline, and malignant variants from different sites. The sequences of c-kit gene were also evaluated in typical smooth muscle tumors, schwannomas and other tumors, especially in 4 adenocarcinmas accompanied by GISTs.
Our series of 52 benign, borderline, and malignant GISTs showed that 96.2% of the GISTs expressed CD117 and only some of the GISTs had mutations. The results indicated a considerable proportion of GISTs expressed CD117 with no mutation. In other words, most of c-kit mutations did not affect the proportion of GISTs with CD117 expression. Mutations in exon 11 of the c-kit gene were observed in 56% of malignant cases. In constrast, only 2 borderline GISTs (10.5%) showed mutations, and no benign GISTs showed mutations, suggesting that the mutations of exon 11 of the c-kit gene may represent a genotypic marker with a correlation to malignancy.
Lasota and colleagues[13] reported the mutation of exons 9 and 13, but the mutation rate seemed to be very low, and they only detected the small portion (8%) of mutations of GISTs in exons 9 and 13, which did not have mutations in exon 11 of the c-kit gene. In our series, no mutations were detected. The result also indicated the rates of mutations in other regions of the c-kit gene were very low.
Our present results were in accordance with those previously reported[14-16]. In hot spot region of exon 11 involving codons 550-560, point mutation and frame deletion were mainly detected at these codons, and 2 cases showed homozygous deletions.
In addition to the change at codons of 550-560, however, the duplication mutation fashion was found in 3 cases to cluster at codons 570 to 585, which was rarely detected in the previously reports.
Analysis of c-kit mutation pattern in relapse lesions showed the persistence of the mutation that remained identical in the primary lesion. Therefore, the c-kit mutation pattern could be tumor-specific.
C-kit mutations were never observed in smooth muscle tumors or nerve sheath tumors or other soft tissue tumors. Furthermore, one adenocarcinoma accompanied by mutation-positive GIST was the wild-type c-kit gene. Therefore, c-kit mutations may also represent a genotypic lineage marker for GISTs.
Previous and present molecular studies have revealed the gain-of-function mutations of the c-kit gene could be observed in mast cell neoplasms[17,18], human germ cell tumors[19] and GISTs, but the location of c-kit mutations differs in them. The particular aspartic acid in the tyrosine kinase domain would changes into valine in mast cell neoplasms, The aspartic acid at amino acid position 816 would change into histidine, whereas deletion, point mutation, or both in the juxtamembrane domain were observed in GISTs mutations. Specially, the mutations were located within codons 550 to 560 in exon 11, and rare mutations are detectable in other domains of the c-kit gene, such as exons 9, 13. Clustering of c-kit mutations in the same region of GISTs and in different region with mast cell neoplasm supported their specific biological significance.
The occurrence of activating mutations involving exon 11 of the c-kit gene in sporadic GIST and the association of exon 11 germline mutations in familial GISTs indicated they were important in GIST’s tumorigenesis[20]. Especially, c-kit mutations could be detected only in some of GISTs[21,22], so the value of the c-kit gene mutation in GISTs inspired the researchers, but there are no concordant conclusions, reports regarding the frequency of mutations in exon 11 varied widely, and prognostic significance of c-kit mutations with respect to the association between exon 11 mutations and malignant histology and/or aggressive malignant behavior were conflicting, and the reported frequency of these mutations varied over a wide range[23-29]. Taniguchi and colleagues[23] found that c-kit mutation-positive GIST were worse than c-kit mutation-negative GISTs. But other studies showed that c-kit exon 11 mutation analysis did not correlate well with histological assessment of malignant potential, and could not be regarded as a reliable objective marker for poor prognosis in GISTs.
Some malignant GISTs lack of exon 11 mutation suggested that mutations in exon 11 of c-kit gene were not the only mechanisms related to malignancy and there must exist other molecular mechanisms[30-33], which may include mutations in other regions of the c-kit gene. But previous report and our study indicated other region mutation of the c-kit gene was few. Recently, Heihrich and colleagues[34] have made an important finding in GISTs, they reported that 35% (14/40) of GISTs lacking c-kit mutations had intragenic activation mutations in tyrosine kinase, platelet-derived growth factor (PDGFRA). C-kit gene and PDGFRA mutations appeared to be alternative and mutually exclusive in GISTs, further study is needed to understand the role of PDGFRA in GISTs.
In summary, the results showed a consistent c-kit expression in GISTs with or without detectable mutations. The mutations occurred more preferentially in malignant GISTs than versus in benign GISTs, but not in smooth muscle tumor and other tumors. These observations suggest that mutations in exon 11 of the c-kit gene might represent useful molecular genetic markers for malignant GISTs and help to differentiate GIST from other tumors. Whether the c-kit gene mutation can indicate bad prognosis of malignant GISTs needs to be further studied.
We thank Dr. Lu X,Y. and Dr. Yu M. for kindly providing the cases.
Edited by Wang XL, Xu FM
| 1. | Chan JK. Mesenchymal tumors of the gastrointestinal tract: a paradise for acronyms (STUMP, GIST, GANT, and now GIPACT), implication of c-kit in genesis, and yet another of the many emerging roles of the interstitial cell of Cajal in the pathogenesis of gastrointestinal diseases? Adv Anat Pathol. 1999;6:19-40. |
| 2. | Tworek JA, Goldblum JR, Weiss SW, Greenson JK, Appelman HD. Stromal tumors of the abdominal colon: a clinicopathologic study of 20 cases. Am J Surg Pathol. 1999;23:937-945. |
| 3. | Sircar K, Hewlett BR, Huizinga JD, Chorneyko K, Berezin I, Riddell RH. Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am J Surg Pathol. 1999;23:377-389. |
| 4. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. |
| 5. | Moskaluk CA, Tian Q, Marshall CR, Rumpel CA, Franquemont DW, Frierson HF. Mutations of c-kit JM domain are found in a minority of human gastrointestinal stromal tumors. Oncogene. 1999;18:1897-1902. |
| 6. | Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum Pathol. 1999;30:1213-1220. |
| 7. | Seidal T, Edvardsson H. Expression of c-kit (CD117) and Ki67 provides information about the possible cell of origin and clinical course of gastrointestinal stromal tumours. Histopathology. 1999;34:416-424. |
| 8. | Robinson TL, Sircar K, Hewlett BR, Chorneyko K, Riddell RH, Huizinga JD. Gastrointestinal stromal tumors may originate from a subset of CD34-positive interstitial cells of Cajal. Am J Pathol. 2000;156:1157-1163. |
| 9. | Miettinen M, Sobin LH, Sarlomo-Rikala M. Immunohistochemical spectrum of GISTs at different sites and their differential diagnosis with a reference to CD117 (KIT). Mod Pathol. 2000;13:1134-1142. |
| 10. | Miettinen M, Furlong M, Sarlomo-Rikala M, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the rectum and anus: a clinicopathologic, immunohistochemical, and molecular genetic study of 144 cases. Am J Surg Pathol. 2001;25:1121-1133. |
| 11. | Smithey BE, Pappo AS, Hill DA. C-kit expression in pediatric solid tumors: a comparative immunohistochemical study. Am J Surg Pathol. 2002;26:486-492. |
| 12. | Hornick JL, Fletcher CD. Immunohistochemical staining for KIT (CD117) in soft tissue sarcomas is very limited in distribution. Am J Clin Pathol. 2002;117:188-193. |
| 13. | Lasota J, Wozniak A, Sarlomo-Rikala M, Rys J, Kordek R, Nassar A, Sobin LH, Miettinen M. Mutations in exons 9 and 13 of KIT gene are rare events in gastrointestinal stromal tumors. A study of 200 cases. Am J Pathol. 2000;157:1091-1095. |
| 14. | Allander SV, Nupponen NN, Ringnér M, Hostetter G, Maher GW, Goldberger N, Chen Y, Carpten J, Elkahloun AG, Meltzer PS. Gastrointestinal stromal tumors with KIT mutations exhibit a remarkably homogeneous gene expression profile. Cancer Res. 2001;61:8624-8628. |
| 15. | Miettinen M, Sarlomo-Rikala M, Sobin LH, Lasota J. Esophageal stromal tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 17 cases and comparison with esophageal leiomyomas and leiomyosarcomas. Am J Surg Pathol. 2000;24:211-222. |
| 16. | Wardelmann E, Neidt I, Bierhoff E, Speidel N, Manegold C, Fischer HP, Pfeifer U, Pietsch T. c-kit mutations in gastrointestinal stromal tumors occur preferentially in the spindle rather than in the epithelioid cell variant. Mod Pathol. 2002;15:125-136. |
| 17. | Jordan JH, Fritsche-Polanz R, Sperr WR, Mitterbauer G, Födinger M, Schernthaner GH, Christian Bankl H, Gebhart W, Chott A, Lechner K. A case of 'smouldering' mastocytosis with high mast cell burden, monoclonal myeloid cells, and C-KIT mutation Asp-816-Val. Leuk Res. 2001;25:627-634. |
| 18. | Sotlar K, Escribano L, Landt O, Möhrle S, Herrero S, Torrelo A, Lass U, Horny HP, Bültmann B. One-step detection of c-kit point mutations using peptide nucleic acid-mediated polymerase chain reaction clamping and hybridization probes. Am J Pathol. 2003;162:737-746. |
| 19. | Tian Q, Frierson HF, Krystal GW, Moskaluk CA. Activating c-kit gene mutations in human germ cell tumors. Am J Pathol. 1999;154:1643-1647. |
| 20. | Maeyama H, Hidaka E, Ota H, Minami S, Kajiyama M, Kuraishi A, Mori H, Matsuda Y, Wada S, Sodeyama H. Familial gastrointestinal stromal tumor with hyperpigmentation: association with a germline mutation of the c-kit gene. Gastroenterology. 2001;120:210-215. |
| 21. | Lasota J, Jasinski M, Sarlomo-Rikala M, Miettinen M. Mutations in exon 11 of c-Kit occur preferentially in malignant versus benign gastrointestinal stromal tumors and do not occur in leiomyomas or leiomyosarcomas. Am J Pathol. 1999;154:53-60. |
| 22. | Miettinen M, Sarlomo-Rikala M, Sobin LH, Lasota J. Gastrointestinal stromal tumors and leiomyosarcomas in the colon: a clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases. Am J Surg Pathol. 2000;24:1339-1352. |
| 23. | Taniguchi M, Nishida T, Hirota S, Isozaki K, Ito T, Nomura T, Matsuda H, Kitamura Y. Effect of c-kit mutation on prognosis of gastrointestinal stromal tumors. Cancer Res. 1999;59:4297-4300. |
| 24. | Rubin BP, Singer S, Tsao C, Duensing A, Lux ML, Ruiz R, Hibbard MK, Chen CJ, Xiao S, Tuveson DA. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res. 2001;61:8118-8121. |
| 25. | Corless CL, McGreevey L, Haley A, Town A, Heinrich MC. KIT mutations are common in incidental gastrointestinal stromal tumors one centimeter or less in size. Am J Pathol. 2002;160:1567-1572. |
| 26. | Miettinen M, El-Rifai W, H L Sobin L, Lasota J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol. 2002;33:478-483. |
| 27. | Andersson J, Sjögren H, Meis-Kindblom JM, Stenman G, Aman P, Kindblom LG. The complexity of KIT gene mutations and chromosome rearrangements and their clinical correlation in gastrointestinal stromal (pacemaker cell) tumors. Am J Pathol. 2002;160:15-22. |
| 28. | Miettinen M, Kopczynski J, Makhlouf HR, Sarlomo-Rikala M, Gyorffy H, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the duodenum: a clinicopathologic, immunohistochemical, and molecular genetic study of 167 cases. Am J Surg Pathol. 2003;27:625-641. |
| 29. | O'Leary T, Ernst S, Przygodzki R, Emory T, Sobin L. Loss of heterozygosity at 1p36 predicts poor prognosis in gastrointestinal stromal/smooth muscle tumors. Lab Invest. 1999;79:1461-1467. |
| 30. | O'leary T, Berman JJ. Gastrointestinal stromal tumors: answers and questions. Hum Pathol. 2002;33:456-458. |
| 31. | El-Rifai W, Sarlomo-Rikala M, Andersson LC, Knuutila S, Miettinen M. DNA sequence copy number changes in gastrointestinal stromal tumors: tumor progression and prognostic significance. Cancer Res. 2000;60:3899-3903. |
| 32. | Heinrich MC, Rubin BP, Longley BJ, Fletcher JA. Biology and genetic aspects of gastrointestinal stromal tumors: KIT activation and cytogenetic alterations. Hum Pathol. 2002;33:484-495. |
| 33. | Kim NG, Kim JJ, Ahn JY, Seong CM, Noh SH, Kim CB, Min JS, Kim H. Putative chromosomal deletions on 9P, 9Q and 22Q occur preferentially in malignant gastrointestinal stromal tumors. Int J Cancer. 2000;85:633-638. |