Published online Apr 15, 2004. doi: 10.3748/wjg.v10.i8.1222
Revised: November 27, 2003
Accepted: December 16, 2003
Published online: April 15, 2004
AIM: To explore how to trigger an HLAI-restricted CD8+ T cell response to exogenously synthesized polypeptides in vivo.
METHODS: Three mimetic therapeutic polypeptides based on the immunodominant CTL epitope of HBcAg, the B- epitope of HBV PreS2 region and a common T helper sequence of tetanus toxoid were designed and synthesized with Merrifield’s solid-phase peptide synthesis method. Their immunological properties of inducing TH1 polarization, CD8+ HBV-specific CTL expansion and CD8+ T cell mediated cytotoxicity were investigated in HLA-A2 transgenic mice.
RESULTS: Results demonstrated that the mimetic polypeptides comprised of the immunodominant CTL, B-, and T helper epitopes could trigger specifically and effectively vigorous CD8+ HBV-specific CTL-mediated cytotoxicity and TH1 polarization of T cells in HLA-A2 transgenic mice.
CONCLUSION: A designed universal T helper plus B-epitopes with short and flexible linkers could dramatically improve the immunogenicity of CTL epitopes in vivo. And that the mimetic therapeutic peptides based on the reasonable match of the above CTL, B- and T helper epitopes could be a promising therapeutic peptide vaccine candidate against HBV infection.
- Citation: Shi TD, Wu YZ, Jia ZC, Zhou W, Zou LY. Therapeutic polypeptides based on HBcAg18-27 CTL epitope can induce antigen-specific CD8+ CTL-mediated cytotoxicity in HLA-A2 transgenic mice. World J Gastroenterol 2004; 10(8): 1222-1226
- URL: https://www.wjgnet.com/1007-9327/full/v10/i8/1222.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i8.1222
At present, the commercial vaccines against HBV infection are mainly based on humoral responses which can prevent the virus infection, but can rarely interrupt the intracellular infection or lead to the infected cell clearance. As in other infections with noncytopathic viruses, an MHC class I-restricted cytotoxic T lymphocytes (CTLs) response to endogenous HBV antigens is believed to be the major determinant for infected cell clearance, and HBV-specific CTL-mediated cytotoxicity plays the key role in controlling HBV infection and in the clearance of infected cells[1-8].
Natural HBV antigens contain generally inappropriate epitopes which could elicit TH1/TH2 disequilibrium, immune deviation or immune deficiency, and the conserved amino acid sequences might also interfere with intercellular communication. Thereby some viruses may evade the immune defence and present consistently in hepatocytes, and result in chronic hepatitis, liver cirrhosis, and even hepatocellular carcinoma[9-19]. Thus new generations of therapeutic vaccines should induce CTL responses different from that induced by natural virus infection, and at the same time hold the specificity of HBV antigens. According to reports in recent years, effective protection relys on the appropriate match of a set of epitopes[19,20]. Thus, natural antigens should be redesigned or modified on the basis of immunodominant epitopes.
In this study, we chose the immunodominant CTL epitope of HBcAg and the B cell epitope of HBV PreS2 region, and introduced them into the common T helper epitope of tetanus toxoid to strengthen the Th response. Three mimetic peptides based on the above epitopes were initially designed and synthesized, and their immunological functions of inducing TH1 polarization, CD8+ HBV-specific CTL expansion and CD8+ HBV-specific CTL-mediated cytotoxicity were investigated in HLA-A2 transgenic mice. We aimed to explore how to trigger an HLAI-restricted CD8+ T cell response to exogenously synthesized polypeptides in vivo, and to find a rational strategy of stimulating the HBV specific CTL response in vivo and to break to some extent the immune tolerance to HBV antigens.
Inbred male and female HLA-A2 transgenic mice (H-2Kb) aging 6-8 wk were purchased from Jackson Laboratory, USA. Amino acids used for peptide synthesis were purchased from PE & ACT companies. Na251 GrO4 for target cell labeling in standard 51Gr release assay was from New England Nuclear (NENTM), Boston, USA. There were also materials used in this study as the following: RPMI1640 medium (Gibco), fetal calf serum (FCS) (HyClone), chimeric HLA-A2Kb tetramer kit (ProImmune, UK) and murine IFN-γ ELISpot kit (Diaclone, France).
Preparation of mimetic therapeutic polypeptides The immunodominant B- and CTL epitopes of HBV pre-S2 and HBcAg were identified on the basis of the HLA-A2.1 binding motifs[21]. Mimetic polypeptides were calculated and sieved using computerized molecular design methods. Peptide1 was determined as the immunodominant HBcAg18-27 CTL epitope peptide (FLPSDFFPSV), to the N-termini of which linked the common T helper sequence of tetanus toxoid with a linker of “-Gly-Gly-Gly-” as peptide2 (QYIKANSKFIGITE GGG FLPSDFFPSV). The common T helper epitope of tetanus toxoid and the Pre-S2 B-epitope were linked to the N- and C-termini of the HBcAg18-27 sequence respectively with the linker of“-Ala-Ala-Ala-” and “-Gly-Gly-Gly-” as peptide3 (QYIKANSKFIGITE AAA FLPSDFFPSVGGG DPRVRGLYFPA). Melanoma associated MART-127-35 CTL epitope peptide (AAGIGILTV) was used as irrelevant control.
The above peptide antigens were synthesized with the Merrifield’s solid-phase peptide synthesis method (PE431A synthesizer), purified by RP-HPLC (WATERS 600) and analyzed by MS/MS (API 2000). All peptides with a purity over 95% were disolved in DMSO with the concentration of 10 mg/mL and preserved at -70 °C.
Immunization of mice Mimetic polypeptides in DMSO were diluted with 0.02 mol/L, pH 7.2 phosphate buffered saline (PBS) and emulsified respectively with complete Freund’s adjuvants (CFA) and incomplete Freund’s adjuvants (IFA). HLA-A2 transgenic mice were separated into 5 groups with 3 in each. The mice were injected subcutaneously in the 2 flanks and in the hind footpads first with peptide1, 2, 3 and then irrelevant control peptide in CFA at 500 μg/kg dosage respectively. Two weeks later, the mice were immunized weekly with the same antigens at IFA in 250 μg/kg dosage for 4 times. The mice injected with PBS alone were used as negative controls.
Two weeks after the last time of immunization, the mice were killed, the splenocytes were separated and suspended in RPMI1640 medium supplemented with 100 mL/L FCS and used as fresh samples[19].
TH1 polarization assay For the assay of TH1 polarization induced by mimetic peptides, mouse IFN-γ ELISPOT kit was used. Briefly[19,22], 96-well PVDF membrane-bottomed plates were coated with capture anti-mouse IFN-γ mAb at 4 °C overnight. Fresh splenocytes were added to triplicated wells at 5 × 103/well in the presence of 10 μg/mL relative mimetic antigens and the plates were incubated for 15 h at 37 °C in 50 mL/L CO2. At the end of incubation, cells were washed off and a second biotinylated anti-IFN-γ mAb was added, followed by streptavidin-alkaline phosphatase conjugate and substrates. After the plates were washed with tap water and dried overnight, spots were counted under a stereomicroscope. The number of TH1 polarized cells (peptide-specific CD8+ T cells), expressed as IFN-γ secreting cells(ISC) /106 lymphocytes, was calculated after subtracting negative control values. Results of samples were considered as positive if above the mean by three standard deviations and with a cut off of 50 ISC/106 lymphocytes above mean background.
Cytotoxicity assay Peptide-specific CTL lines were primed as follows: The fresh splenocytes were plated at a concentration of 2 × 105/mL in 24-well microplates (2 mL/well) in PRMI1640 medium supplemented with 100 mL/L FCS and 30 U/mL murine IL-2 and in the presence of 10 μg/mL corresponding mimetic peptides respectively, with an exception of the lymphocytes from negative controls which were just cultivated in medium supplemented with 100 mL/L FCS and 30 U/mL murine IL-2. Five days after stimulation, cells were harvested, and used as fresh effectors.
CTL-mediated cytotoxicity was detected by a standard 4 h 51Cr release assay[18,19,23,24]. P815 cells were used as targets and preincubated with HBcAg18-27 peptide 2 h before use. The 1 × 106 target cells were labeled with 3.7 × 106 Bq Na251GrO4 in 0.8 mL RPMI1640 medium supplemented with 150 mL/L FCS for 60 min at 37 °C, and then washed 3 times before the addition of effectors. Various concentrations of effector cells were mixed with 1 × 104 targets at effector/target(E/T) ratios of 12.5, 25, 50 and 100 in 200 μL of culture medium in 96-well V-bottomed microplate in triplicates. The microplate was centrifuged for 3 min at 500 r/min, and then incubated for 4 h at 37 °C in 50 mL/L CO2. After the incubation terminated, 100 μL/well of supernatants was harvested and counted on a γ-counter. Percentage of target cell specific lysis was determined as: [(average sample counts - average spontaneous counts)/ (average maximum counts - average spontaneous counts)] × 100%. Maximum and spontaneous counts were measured using supernatants from wells receiving 1 mol/L HCl or culture medium alone, respectively. In all experiments, spontaneous counts should be less than 30% of maximum counts. CTL responses were considered positive if exceeded the mean of specific lysis caused by irrelevant mimetic antigen (MART-127-35) by 3 standard deviations and by 10%.
Chimeric HLA-A2Kb tetramer binding kit was used to quantify the HBcAg18-27-specific CD8+ T cells[24-26]. A part of the fresh splenocytes separated from mice killed were washed with PBS, counted, suspended in PBS and separated equally into different tubes in 1.0 mL of each. The cells were stained with 25 ng/μL of tetramers for 20 min at 37 °C, and then washed and stained with anti-mouse CD8 mAb for 20 min at room temperature. All samples were collected, washed twice, dissolved into 300 μL of PBS and FACS-sorted on a FACstar (Beckton-Dicknson) with Cell Quest software. Results were expressed as percentages of tetramer-binding cells in the murine CD8+ T cell population. A total of 5 × 105 events were acquired in each analysis. Results were considered as positive for tetramer-binding cells when above the mean caused by irrelevant mimetic antigen (MART-127-35) and above 0.1% CD8+ T cells.
All data were expressed as mean ± SD. Statistical analysis was performed using a two-tailed Student’s t test.
When the immunization terminated, the mice were killed and the spleen lymphocytes were used for TH1 polarization assay with an IFN-γ ELISPOT method. Spots of IFN-γ secreting cells generated could be observed in each of the mimetic peptides immunized groups. The negative control values of peptide-specific CD8+ T cells were on average 250 ± 138 ISC/106 lymphocytes. The most vigorous peptide-specific CD8+ T cells magnification was produced in peptide3 immunized mice with approximately 8 050 ± 1 233.8 ISC/106 lymphocytes generated. In peptide1 and 2 immunization groups, the peptide-specific CD8+ T cells induced were 1 150 ± 236.7 and 1 367 ± 231 ISC/106 lymphocytes respectively. And also 1 135 ± 312 ISC/106 lymphocytes were generated in MART-127-35 CTL epitope peptide immunized mice (Table 1).
When the mice were killed, the fresh splenocytes were separated and peptide-specific CTL lines were generated ex vivo. The CTL-mediated cytotoxicity induced was tested by standard 4 h 51Cr release assay against HBcAg18-27 peptide preincubated P815 targets. Data demonstrated that all the three mimetic polypeptides based on HBcAg18-27 CTL epitope could induce positive HBV-specific CTL response, among which peptide3 induced the most vigorous CTL activity, and as high as (55.3 ± 10.1)% targets lysis was observed at E/T = 100. The percentages of targets lysed in peptide1 and 2 immunization groups were dramatically lower than in peptide 3 group (P < 0.01) and showed statistically no difference between. The spleen lymphocytes from irrelevant peptide control and negative control mice showed no specific target cell lysis activity (Table 2).
| E/T ratios | Percentage of specific cell lysis (%) | ||||
| Peptide1d | Peptide2 | Peptide3 | MART-127-35 | Negative controls | |
| 12.5 | 18.5 ± 4.3d | 21.1 ± 6.3d | 33.7 ± 7.2db | 3.7 ± 0.7 | 5.5 ± 0.9 |
| 2 5 | 22.9 ± 5.7d | 28.5 ± 6.6d | 40.5 ± 8.9db | 6.3 ± 0.9 | 4.4 ± 0.9 |
| 5 0 | 29.3 ± 7.0d | 30.5 ± 8.8d | 50.3 ± 11.3db | 7.1 ± 1.1 | 7.7 ± 1.2 |
| 100 | 32.6 ± 10.1d | 33.1 ± 8.2d | 55.3 ± 10.1db | 8.4 ± 1.3 | 9.2 ± 1.3 |
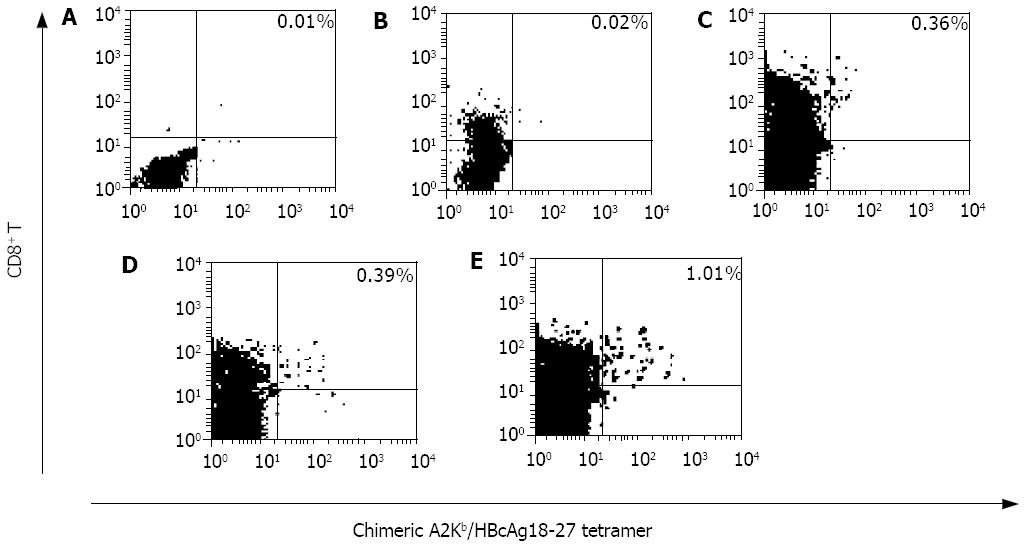
After the immunization terminated, the HBcAg18-27-specific CD8+ T cells induced in vivo were quantified using chimeric A2Kb/HBcAg18-27 tetramer-binding assay. No HbcAg positive CD8+ T cells could be detected in the spleen lymphocytes from the negative control mice and the mice immunized with MART-127-39 peptide, and the tetramer staining was lower than background (0.02%). In splenocytes from the mice immunized with peptide1, 2 and 3, the frequencies of A2Kb/HBcAg18-27·CD8 positive T cells were respectively 0.36% (3 600/106 lymphocytes), 0.39% (3 900/106 lymphocytes), and 1.01% (10 100/106 lymphocytes). It showed no statistical difference between the effects induced by peptide1 and peptide2, and the immunogenicity of them was dramatically weaker than that of peptide3 (Figure 1).
HBV-specific CD8+ cytotoxic T cells play a critical role in viral clearance. Low HBV-specific CTL responses in chronic HBV infection may favor the persistence of virus, whereas stimulation and expansion of HBV-specific CTL activity may assist elimination of HBV infection[1-8]. Natural HBV antigens contain some inappropriate epitopes and conserved amino acid sequences which might induce inappropriate immune responses and result in hepatic pathology and lesions. Hence new generations of therapeutic vaccines should be designed on the basis of immunodominant epitopes which could induce CTL responses different from that induced by natural virus infection, and at the same time hold the specificity of HBV antigens. As in other infections with noncytopathic viruses, helper T cells control the intensity of CD8+ T-cell responses and helper T-cell responses might be compromised in chronic carriers of HBV[27-32]. In this paper, we chose the immunodominant B cell epitope of HBV PreS2 region and the CTL epitope of HBcAg, and introduced them into the common T helper epitope of tetanus toxoid to strengthen the Th response.Three mimetic peptides based on the above epitopes were initially designed and synthesized, and their immunological properties of inducing TH1 polarization, CD8+ T-cell expansion and CTL-mediated cytotoxicity were primarily investigated in HLA-A2 transgenic mice.
After immunization, the mice were killed and the splenocytes were separated, a direct tetramer-binding assay was used to detect the frequencies of HBcAg18-27-specific CD8+ T cells, the results varied according to the peptides used. The highest frequency was from peptide3 immunized mice splenocytes. No statistical difference was observed between the frequencies of HBcAg18-27-specific CD8+ T cells augmented in peptide1 and peptide2 mice groups, which indicated that the immunogenicity of short CTL epitope peptides in vivo could not be improved dramatically simply by introduction of a universal T helper epitope, and that by our reasonable match of the above immunodominant CTL, B-, T helper epitopes and linkers, the immunogenicity of HBcAg18-27 CTL epitope of inducing HBV-specific CD8+ T cell expansion in vivo was dramatically improved.
The tetramer-binding assay detects only the number of T cells with an appropriate TCR but not their function[24,33], so a chronium release assay and IFN-γ ELISpot assay were used to detect the immune functions of the mimetic antigens designed. And a highly significant correlation was found between the frequencies of HBcAg18-27-specific CD8+ T cells and the functions of responding splenocytes from the mice. The three mimetic polypeptides designed could induce TH1 polarization of spleen lymphocytes and generate cytotoxicity in mice, among which peptide3 with the immunodominant B-, CTL and T helper epitopes was the most potent, peptide 1 and 2 could also produce the above immune functions but not as efficiently. After introducing T helper epitope into HBcAg18-27, the CD8+ CTL frequency was not remarkably improved, and cytotoxic activity remained low, suggesting that the help provided by this conformation was not sufficient to drive proliferation of CTL, and their differentiation into mature killer cells. The comparatively higher immunogenicity of peptide3 might rely on its molecular structure: the introduction of T helper and B-epitopes, the design of short linkers “Ala-Ala-Ala” and “Gly-Gly-Gly”, and the reasonable match. The designed linker was proved to be highly flexible and might act as “hinges”. We surmise that the peptide be recognized by MHC-I and II restricted molecules, and be presented to CD4+ T cells and CD8+ T cells, and ultimately T helper and Tc cells be activated and functioned interactively. It indicated that introducing short and flexible “hinges” and “Th+B” epitopes into short CTL epitope peptides might dramatically improve the peptide’s immunogenicity and the ability of being presented to APCs in vivo. The results also demonstrated that designed peptide3 was highly immunogenic and HBV-specific in vivo, and might be a potential candidate for the therapeutic vaccine designed against hepatitis B.
Little knowledge is known so far on the molecular mechanisms of the in vitro and in vivo functions of the peptides[33-38]. In our opinion, in vivo induction of cytotoxic activity relys on the efficient presentation by APCs, and the crucial point is how to improve the antigenicity of short peptides so to meet the needs for efficient antigen presentation in vivo. Thus to redesign or modify the linear short peptides on the basis of immunodominant epitopes, change their molecular properties to meet the needs of antigen presentation, and stimulate the direct recognization of the peptides by TH/Tc cells may be a promising approach to this problem.
Co-correspondents: Tong-Dong Shi and Yu-Zhang Wu
Edited by Zhu LH and Xu FM
| 1. | Stober D, Trobonjaca Z, Reimann J, Schirmbeck R. Dendritic cells pulsed with exogenous hepatitis B surface antigen particles efficiently present epitopes to MHC class I-restricted cytotoxic T cells. Eur J Immunol. 2002;32:1099-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Wei J, Wang YQ, Lu ZM, Li GD, Wang Y, Zhang ZC. Detection of anti-preS1 antibodies for recovery of hepatitis B patients by immunoassay. World J Gastroenterol. 2002;8:276-281. [PubMed] |
| 3. | Storni T, Lechner F, Erdmann I, Bächi T, Jegerlehner A, Dumrese T, Kündig TM, Ruedl C, Bachmann MF. Critical role for activation of antigen-presenting cells in priming of cytotoxic T cell responses after vaccination with virus-like particles. J Immunol. 2002;168:2880-2886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Wan Y, Wu Y, Bian J, Wang XZ, Zhou W, Jia ZC, Tan Y, Zhou L. Induction of hepatitis B virus-specific cytotoxic T lymphocytes response in vivo by filamentous phage display vaccine. Vaccine. 2001;19:2918-2923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Böcher WO, Dekel B, Schwerin W, Geissler M, Hoffmann S, Rohwer A, Arditti F, Cooper A, Bernhard H, Berrebi A. Induction of strong hepatitis B virus (HBV) specific T helper cell and cytotoxic T lymphocyte responses by therapeutic vaccination in the trimera mouse model of chronic HBV infection. Eur J Immunol. 2001;31:2071-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Blackman MA, Rouse BT, Chisari FV, Woodland DL. Viral immunology: challenges associated with the progression from bench to clinic. Trends Immunol. 2002;23:565-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Huang J, Cai MY, Wei DP. HLA class I expression in primary hepatocellular carcinoma. World J Gastroenterol. 2002;8:654-657. [PubMed] |
| 8. | Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, Chisari FV. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 764] [Article Influence: 34.7] [Reference Citation Analysis (1)] |
| 9. | Rabe C, Pilz T, Klostermann C, Berna M, Schild HH, Sauerbruch T, Caselmann WH. Clinical characteristics and outcome of a cohort of 101 patients with hepatocellular carcinoma. World J Gastroenterol. 2001;7:208-215. [PubMed] |
| 10. | Kessler JH, Beekman NJ, Bres-Vloemans SA, Verdijk P, van Veelen PA, Kloosterman-Joosten AM, Vissers DC, ten Bosch GJ, Kester MG, Sijts A. Efficient identification of novel HLA-A(*)0201-presented cytotoxic T lymphocyte epitopes in the widely expressed tumor antigen PRAME by proteasome-mediated digestion analysis. J Exp Med. 2001;193:73-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 194] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 11. | Ma CH, Sun WS, Tian PK, Gao LF, Liu SX, Wang XY, Zhang LN, Cao YL, Han LH, Liang XH. A novel HBV antisense RNA gene delivery system targeting hepatocellular carcinoma. World J Gastroenterol. 2003;9:463-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Sette AD, Oseroff C, Sidney J, Alexander J, Chesnut RW, Kakimi K, Guidotti LG, Chisari FV. Overcoming T cell tolerance to the hepatitis B virus surface antigen in hepatitis B virus-transgenic mice. J Immunol. 2001;166:1389-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Engler OB, Dai WJ, Sette A, Hunziker IP, Reichen J, Pichler WJ, Cerny A. Peptide vaccines against hepatitis B virus: from animal model to human studies. Mol Immunol. 2001;38:457-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Kakimi K, Isogawa M, Chung J, Sette A, Chisari FV. Immunogenicity and tolerogenicity of hepatitis B virus structural and nonstructural proteins: implications for immunotherapy of persistent viral infections. J Virol. 2002;76:8609-8620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395-1406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 925] [Cited by in RCA: 901] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 16. | Kurts C, Miller JF, Subramaniam RM, Carbone FR, Heath WR. Major histocompatibility complex class I-restricted cross-presentation is biased towards high dose antigens and those released during cellular destruction. J Exp Med. 1998;188:409-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 235] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 17. | Wiesmüller KH, Bessler WG, Jung G. Solid phase peptide synthesis of lipopeptide vaccines eliciting epitope-specific B-, T-helper and T-killer cell response. Int J Pept Protein Res. 1992;40:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Zhou HC, Xu DZ, Wang XP, Zhang JX, Huang Y, Yan YP, Zhu Y, Jin BQ. Identification of the epitopes on HCV core protein recognized by HLA-A2 restricted cytotoxic T lymphocytes. World J Gastroenterol. 2001;7:583-586. [PubMed] |
| 19. | Guan XJ, Guan XJ, Wu YZ, Jia ZC, Shi TD, Tang Y. Construction and characterization of an experimental ISCOMS-based hepatitis B polypeptide vaccine. World J Gastroenterol. 2002;8:294-297. [PubMed] |
| 20. | Chaiken IM, Williams WV. Identifying structure-function relationships in four-helix bundle cytokines: towards de novo mimetics design. Trends Biotechnol. 1996;14:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Livingston BD, Crimi C, Fikes J, Chesnut RW, Sidney J, Sette A. Immunization with the HBV core 18-27 epitope elicits CTL responses in humans expressing different HLA-A2 supertype molecules. Hum Immunol. 1999;60:1013-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Sun Y, Iglesias E, Samri A, Kamkamidze G, Decoville T, Carcelain G, Autran B. A systematic comparison of methods to measure HIV-1 specific CD8 T cells. J Immunol Methods. 2003;272:23-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Wang FS, Liu MX, Zhang B, Shi M, Lei ZY, Sun WB, Du QY, Chen JM. Antitumor activities of human autologous cytokine-induced killer (CIK) cells against hepatocellular carcinoma cells in vitro and in vivo. World J Gastroenterol. 2002;8:464-468. [PubMed] |
| 24. | Cederbrant K, Marcusson-Ståhl M, Condevaux F, Descotes J. NK-cell activity in immunotoxicity drug evaluation. Toxicology. 2003;185:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Choi EM, Palmowski M, Chen J, Cerundolo V. The use of chimeric A2K(b) tetramers to monitor HLA A2 immune responses in HLA A2 transgenic mice. J Immunol Methods. 2002;268:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Kuzushima K, Hayashi N, Kudoh A, Akatsuka Y, Tsujimura K, Morishima Y, Tsurumi T. Tetramer-assisted identification and characterization of epitopes recognized by HLA A*2402-restricted Epstein-Barr virus-specific CD8+ T cells. Blood. 2003;101:1460-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Livingston BD, Alexander J, Crimi C, Oseroff C, Celis E, Daly K, Guidotti LG, Chisari FV, Fikes J, Chesnut RW. Altered helper T lymphocyte function associated with chronic hepatitis B virus infection and its role in response to therapeutic vaccination in humans. J Immunol. 1999;162:3088-3095. [PubMed] |
| 28. | Stebbing J, Patterson S, Gotch F. New insights into the immunology and evolution of HIV. Cell Res. 2003;13:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Wu GH, Zhang YW, Wu ZH. Modulation of postoperative immune and inflammatory response by immune-enhancing enteral diet in gastrointestinal cancer patients. World J Gastroenterol. 2001;7:357-362. [PubMed] |
| 30. | Zhu F, Eckels DD. Functionally distinct helper T-cell epitopes of HCV and their role in modulation of NS3-specific, CD8+/tetramer positive CTL. Hum Immunol. 2002;63:710-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Carcelain G, Tubiana R, Samri A, Calvez V, Delaugerre C, Agut H, Katlama C, Autran B. Transient mobilization of human immunodeficiency virus (HIV)-specific CD4 T-helper cells fails to control virus rebounds during intermittent antiretroviral therapy in chronic HIV type 1 infection. J Virol. 2001;75:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Ciavarra RP, Greene AR, Horeth DR, Buherer K, van-Rooijen N, Tedeschi B. Antigen processing of vesicular stomatitis virus in situ. Interdigitating dendritic cells present viral antigens inde-pendent of marginal dendritic cells but fail to prime CD4(+) and CD8(+) T cells. Immunology. 2000;101:512-520. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Maini MK, Boni C, Lee CK, Larrubia JR, Reignat S, Ogg GS, King AS, Herberg J, Gilson R, Alisa A. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med. 2000;191:1269-1280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 651] [Cited by in RCA: 657] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 34. | Li LS, Qu RY, Wang W, Guo H. Significance of changes of gas-trointestinal peptides in blood and ileum of experimental spleen deficiency rats. World J Gastroenterol. 2003;9:553-556. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Li MS, Li PF, He SP, Du GG, Li G. The promoting molecular mechanism of alpha-fetoprotein on the growth of human hepatoma Bel7402 cell line. World J Gastroenterol. 2002;8:469-475. [PubMed] |
| 36. | Billich A. Thymosin alpha1. SciClone Pharmaceuticals. Curr Opin Investig Drugs. 2002;3:698-707. [PubMed] |
| 37. | Gripon P, Rumin S, Urban S, Le Seyec J, Glaise D, Cannie I, Guyomard C, Lucas J, Trepo C, Guguen-Guillouzo C. Infection of a human hepatoma cell line by hepatitis B virus. Proc Natl Acad Sci USA. 2002;99:15655-15660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 926] [Cited by in RCA: 994] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 38. | Li D, Takyar ST, Lott WB, Gowans EJ. Amino acids 1-20 of the hepatitis C virus (HCV) core protein specifically inhibit HCV IRES-dependent translation in HepG2 cells, and inhibit both HCV IRES- and cap-dependent translation in HuH7 and CV-1 cells. J Gen Virol. 2003;84:815-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |