Published online Apr 15, 2004. doi: 10.3748/wjg.v10.i8.1215
Revised: October 14, 2003
Accepted: December 24, 2003
Published online: April 15, 2004
AIM: To investigate the efficacy and tolerability of albendazole and metranidazole treatment in giardiasis.
METHODS: The open comparative randomized trial was carried out prospectively from December 1999 to July 2001 in Duzce City of Turkey. The diagnosis was based on the presence of signs and symptoms compatible with giardiasis including a positive stool examination of giardia cysts or trophozoite. Metranidazole group consisted of 29 patients and was given metranidazole 500 mg, three times a day for 5 d and albendazole group was consisted of 28 patients and was given albendazole 400 mg/d for 5 d.
RESULTS: There were no significant differences in demographical and therapeutical effects and patient’s compliance between both groups. But side effects were seen more in metranidazole group than in albendazole group.
CONCLUSION: Albendazole is as effective as metranidazole in adults’ giardiasis. Albendazole has less side effect potentials than metranidazole in the treatment of giardiasis.
- Citation: Karabay O, Tamer A, Gunduz H, Kayas D, Arinc H, Celebi H. Albendazole versus metronidazole treatment of adult giardiasis: An open randomized clinical study. World J Gastroenterol 2004; 10(8): 1215-1217
- URL: https://www.wjgnet.com/1007-9327/full/v10/i8/1215.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i8.1215
Giardia intestinalis is a protozoan parasite in the small intestine that causes extensive morbidity worldwide. Giardiasis is a hyperendemic disease in households lacking municipal sewer and water in the developing countries[1]. The life cycle of G. intestinalis has 2 forms: the trophozoite and the cyst. As few as 10 cysts may establish infection[2,3]. Giardiasis is currently treated with metronidazole, tinidazole and quinacrine[3]. The adverse effects and treatment failures to some of the currently recommended drugs (particularly 5-nitroimidazoles) for giardia infection have given rise to the need for alternative antigiardial agents. Albendazole is an important alternative drug for treament of giardiasis. In vitro Albendazole inhibits the growth of trophozoites of G.intestinalis and their adhesion to cultured intestinal epithelial cells and disturbs the activity of microtubules and microribbons in the trophozoite’s adhesive disk. The results of giardiasis treatment with albendazole have been confused. A lot of trials were carried out for gairdiasis treatment with albendazole in pediatric groups. Albendazole was found to be effective for pediatric giardiasis patients. Albendazole at a dose of 400 mg per day for 5 d, cured 97 percent of infections in children in Bangladesh[4]. A few studies were about the effects of albendazole on adult intestinal giardiasis. It was ineffective in a study of adult travelers returning from tropical areas[3,5]. In this study we aimed to investigate effect of albendazol on adult giardiasis compared with metronidazole treatment.
Adults with diarrhea at the outpatient clinic of the Department of Infectious Diseases of Social Security Hospital in Duzce/Turkey were screened for enrollment in the study. After informed consent was obtained, a detailed medical history was taken from each patient and physical examination was performed. For the demonstration of trophozoites or cysts in the stool, 3 stool samples were obtained. Patients with diarrhea and G.intestinalis cysts or trophozoites in a sample were eligible for enrollment in the study. Diarrhea was defined as more than 4 times of unformed stools per day. Giardia cysts were identified in fresh faecal material by direct faecal microscopic examination. A stool culture was carried out to identify bacterial causes of diarrhea. Patients with a positive coproculture for bacterial causes of diarrhea were excluded from analysis. All patients had a clinical response, recorded on d 7, 15, and a parasitological response recorded on the basis of examination of 2 stool samples between d 7 and 15 after initation of treatment.
Patients were randomized to receive albendazole 400 mg/d for 5 d or metronidazole 500 mg thrice daily for 5 d.
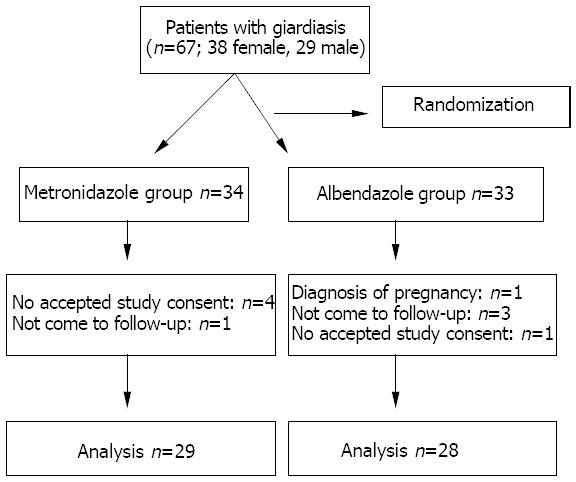
Of the original 67 (38 female and 29 male) patients who were selected, 57 (24 males and 33 females) completed this study in the follow-up period (Figure 1). Twenty-nine patients who received metronidazole (Falgyl®) 500 mg 3 times daily for 5 d. Twenty-eight patients received albendazole (Andazol®) 400 mg /d for 5 d.
All patients were investigated for compliance to treatment, and one of the following requirements should be fulfilled in order to define a case as noncompliance to treatment, namely, failure to attend the controls, not use one or a few of the medicines at the instructed dose and the duration, not use the drug without taking the consent of the doctor.
The study protocol was approved by the locally ethics committee. All patients were informed and agreed to participate in the study.
Gender, age, mean hemoglobin concentration and leukocyte counts were compared between the 2 groups using non-parametric test (Mann-Whitney U test). Difference between the 2 groups was analyzed using chi-square test. We used Epi-info 6.0 (Centers for Disease Control, Atlanta) to perform the analysis and considered P < 0.05 as statistically significant.
Patients receiving or having received antiparasitic drugs during the 10-d prior to commencing the study, patients with fever, pregnant women, mothers who were breast feeding, patients with known hypersensitivity to either albendazole or metronidazole, patients for whom any of the treatments used in the study were contraindicated.
The clinical and demographic findings in the albendazole group and metronidazole group are presented in Table 1.
| Parameter | Metranidazol Group (n=29) | Albendazol Group (n=28) | P |
| Female /Male | 18/11 | 15/13 | # |
| Age (yr) | 38 ± 14 | 41 ± 12 | # |
| Hemoglobin | 13.2 ± 1.5 | 12.7 ± 1.5 | # |
| Leukocyte count | 7 996 ± 2 668 | 8 225 ± 3 016 | # |
| Faecal examination positive | 0 | 0 | NA |
| for cysts or trophozoits on d 7 | |||
| Faecal examination positive for | 0 | 1 | NA |
| cysts or trophozoits on d 15 | |||
| Metal taste | 9 | 0 | NA |
| Anorexia +/- | 18 | 2 | 0.0001 |
| Abdominal pain | 3 | 1 | # |
| After starting treatment | 83 ± 39 | 80 ± 28 | # |
| healing of symptoms (h) | |||
| Advers effects other than anorexia | 8 | 6 | # |
| Non-compliance to treatment | 7 | 5 | # |
No positive giardia cyst was found in the stool samples of both albendozole and metronidazole groups on d 7. But on d 15 one patient in of albendazole group was found to be positive for giardia cyst, while none of the patients in metranidozole group was positive for giardia cyst. Abdominal pain was found in 3 patients of metronidazole group and 1 patient of albendazole group (P > 0.05). Vomiting was seen in 1 patient of metronidazole group and none in albendazole group. Noncompliance to treatment was found in 7 patients of metronidazole group and in 5 patients in albendazole group (P > 0.05). Anorexia was found in 18 patients of metronidazole group and in 2 patients of albendazole group (P < 0.001). Metal taste was determined in nine patients of metronidazole group and none in albendazole group. Records associated with other (headache, abdominal pain, dazedness) adverse effects except anorexia and metal taste were not found to be significantly different between the 2 groups (P > 0.05).
Infections with parasitic helminths and protozoa are important causes of morbidity and mortality worldwide. The protozoan parasite Giardia intestinalis (synonyms: Giardia duodenalis and Giardia lamblia) is recognized as a major cause of diarrheal illness in humans and livestock. It is one of the most important non-viral infectious agents causing diarrheal illness, the infection may be asymptomatic or present with a variety of symptoms such as diarrhea, weight loss, abdominal cramps and failure to thrive. G.intestinalis may attach to small bowel wall but not invade it. Trophozoites may be encysted and shed in faeces for future ingestion by other hosts. Whereas the organism can cause diarrhea and abdominal pain. Some people experienced only a mild self-limiting illness, while others developed a chronic illness lasting for several months. Furthermore, people might be infected without any symptoms, and it has even been suggested that some people could benefit from their carrier state[6,7].
Although Giardia infections resolve spontaneously in 85% of patients within 6 wk, all patients with symptomatic giardiasis should be treated. Metronidazole and quinacrine are the first-line treatment options and are more than 90% effective. Tinidazole, furazolidone, paromomycin, mebendazole, and albendazole have been used as alternative anti-giardial drugs[8]. Cedillo -Rivera et al.[9] investigated the susceptibility of a strain of Giardia lamblia to benzimidazole carbamates, 5-nitroimidazoles, nitrofurans and other drugs. They found that albendazole was the most active compound among the 5-nitroimidazoles tested, ornidazole was the most effective, and tinidazole, metronidazole, secnidazole were less active. Various reports have published the effect of albendazole on gairdiasis. Albendazole was found to be very effective on giardiasis[10]. Misra et al.[10] studied the effect of albendazole and metranidozole on giardiasis in 64 children aged 2-12 years. They concluded that albendazole was proved as effective as metronidazole in the treatment of giardia infection in children with the absence of anorexia. Similarly, another study found albendazole at dose of 400 mg /d for 5 d cured 97% of infections in children in Bangladesh[4]. But, Escobedo et al. investigated in a comparative trial. One hundred and sixty-five Cuban children with confirmed giardiasis were randomized to receive albendazole (400 mg/d for 5 d), chloroquine (10 mg/kg twice daily for 5 d) or tinidazole (50 mg/kg, as a single dose). They found that tinidazole and chloroquine appeared equally effective, curing 91% and 86% of the children treated, respectively, and were significantly better than albendazole, which only cured 62% of the children[11].
In this study we investigated the effect of albendazole and metronidazole on symptomatic adult giardiasis. We did not find any significant difference in demographical properties (gender, age), mean hemoglobins, and mean leukocytes between the 2 groups. Giardia cysts were not found in faecal examination both groups on d 7. But on d 15 after starting treatment, one patient was found to be positive for giardia in albendazole group and none in metronidazole group. We thought that this patient might be reinfected. After starting treatment, 9 patients complained of metal taste in metronidazole group and no patient in albendazole group. Anorexia was found in 18 patients of metronidazole group but only 2 patients complained of anorexia in albendazole group (P < 0.01). In terms of adverse effects, albendazole was found superior to metronidazole. Patients’ compliance was found to be similar in both groups (P > 0.05). We thought that it might be due to a short treatment period (five days). Similarly, chan Del Pino et al.[12] investigated the efficacy and tolerance of albendazol compared with metranidazol, furazolidone, tinidazol and secnidazol in the treatment of giardiasis in 79 children. They concluded that albendazol was as effective as metronidazol, furazolidone, tinidazol and secnidazol, but faster in eradicating Giardia lambila in children and had a better tolerance than metranidazol, furazolidone and tinidazol.
The drug resistance was not an important problem for giardiasis[13-15]. In our country cost of both drugs is similarly. We thought that both drugs can be used in the treatment of giardiasis, because according to our results albendazole is as effective as metronidazole in adult’s giardiasis and albendazole treatment has also less side effects than metronidazole.
The authors would like to thank Dr. Gurhan Konakci (Chief Manager of Social Security Duzce Hospital) for providing his help.
Edited by Wang XL and Xu FM
| 1. | Redlinger T, Corella-Barud V, Graham J, Galindo A, Avitia R, Cardenas V. Hyperendemic Cryptosporidium and Giardia in households lacking municipal sewer and water on the United States-Mexico border. Am J Trop Med Hyg. 2002;66:794-798. [PubMed] |
| 2. | Gardner TB, Hill DR. Treatment of giardiasis. Clin Microbiol Rev. 2001;14:114-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 327] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 3. | Liu LX, Weller PF. Antiparasitic drugs. N Engl J Med. 1996;334:1178-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 86] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Hall A, Nahar Q. Albendazole as a treatment for infections with Giardia duodenalis in children in Bangladesh. Trans R Soc Trop Med Hyg. 1993;87:84-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 63] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Kollaritsch H, Jeschko E, Wiedermann G. Albendazole is highly effective against cutaneous larva migrans but not against Giardia infection: results of an open pilot trial in travellers returning from the tropics. Trans R Soc Trop Med Hyg. 1993;87:689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Vesy CJ, Peterson WL. Review article: the management of Giardiasis. Aliment Pharmacol Ther. 1999;13:843-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Homan WL, Mank TG. Human giardiasis: genotype linked differences in clinical symptomatology. Int J Parasitol. 2001;31:822-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 170] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Tessier JL, Davies G. Giardiasis. Primary. Care Update for OB/GYNS. 1999;6:8-11. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Cedillo-Rivera R, Muñoz O. In-vitro susceptibility of Giardia lamblia to albendazole, mebendazole and other chemotherapeutic agents. J Med Microbiol. 1992;37:221-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Misra PK, Kumar A, Agarwal V, Jagota SC. A comparative clinical trial of albendazole versus metronidazole in children with giardiasis. Indian Pediatr. 1995;32:779-782. [PubMed] |
| 11. | Escobedo AA, Núñez FA, Moreira I, Vega E, Pareja A, Almirall P. Comparison of chloroquine, albendazole and tinidazole in the treatment of children with giardiasis. Ann Trop Med Parasitol. 2003;97:367-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Chan Del Pino M, Cueva Cornejo L, Troyes Rivera L. [Comparative study of albendazole versus nitrofurans and nitroimidazoles in the treatment of giardiasis in children]. Rev Gastroenterol Peru. 1999;19:95-108. [PubMed] |
| 13. | Cruz A, Sousa MI, Azeredo Z, Leite E, Figueiredo de Sousa JC, Cabral M. Isolation, excystation and axenization of Giardia lamblia isolates: in vitro susceptibility to metronidazole and albendazole. J Antimicrob Chemother. 2003;51:1017-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Wright JM, Dunn LA, Upcroft P, Upcroft JA. Efficacy of antigiardial drugs. Expert Opin Drug Saf. 2003;2:529-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Ali SA, Hill DR. Giardia intestinalis. Curr Opin Infect Dis. 2003;16:453-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |