Published online Apr 15, 2004. doi: 10.3748/wjg.v10.i8.1110
Revised: July 2, 2003
Accepted: July 30, 2003
Published online: April 15, 2004
AIM: To investigate the roles of c-Jun N-terminal kinase (JNK) signaling pathway in vitamin E succinate-induced apoptosis in human gastric cancer SGC-7901 cells.
METHODS: Human gastric cancer cell lines (SGC-7901) were treated with vitamin E succinate (VES) at 5, 10, 20 mg/L. Succinic acid and vitamin E were used as vehicle controls and condition medium only as an untreated (UT) control. Apoptosis was observed by 4’, 6-diamidine-2’-phenylindole dihydrochloride (DAPI) staining for morphological changes and by DNA fragmentation for biochemical alterations. Western blot analysis was applied to measure the expression of JNK and phosphorylated JNK. After the cells were transiently transfected with dominant negative mutant of JNK (DN-JNK) followed by treatment of VES, the expression of JNK and c-Jun protein was determined.
RESULTS: The apoptotic changes were observed after VES treatment by DNA fragmentation. DNA ladder in the 20 mg/L VES group was more clearly seen than that in 10 mg/L VES group and was not detected following treatment of UT control, succinate and vitamin E. VES at 5, 10 and 20 mg/L increased the expression of p-JNK by 2.5-, 2.8- and 4.2-fold, respectively. VES induced the phosphorylation of JNK beginning at 1.5 h and produced a sustained increase for 24 h with the peak level at 12 h. Transient transfection of DN-JNK blocked VES-triggered apoptosis by 52%. DN-JNK significantly increased the level of JNK, while decreasing the expression of VES-induced c-Jun protein.
CONCLUSION: VES-induced apoptosis in human gastric cancer SGC-7901 cells involves JNK signaling pathway via c-Jun and its downstream transcription factor.
- Citation: Wu K, Zhao Y, Li GC, Yu WP. c-Jun N-terminal kinase is required for vitamin E succinate-induced apoptosis in human gastric cancer cells. World J Gastroenterol 2004; 10(8): 1110-1114
- URL: https://www.wjgnet.com/1007-9327/full/v10/i8/1110.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i8.1110
Vitamin E is characterized as a fat-soluble membrane antioxidant[1-3]. RRR-α-tocopheryl succinate (vitamin E succinate, VES), a derivative of natural vitamin E, does not possess antioxidant properties unless succinate group is hydrolyzed by specific ester hydrolase. VES has been shown to be a potent growth inhibitor of a variety of malignant cell types in vitro and in vivo, including avian lymphoid cells[4], murine B16 melanoma cells[5] and EL4 T lymphoma cells[6,7], human monoblastic leukemia cells[8], prostate[9,10], breast[11-13] and gastric cancer cells[14,15]. VES has also been shown to suppress tumorigenesis in hamster buccal pouch[16], mouse forestomach[17] and mammary gland[18]. These antitumor effects seem to be selective for tumor cells since VES treatment is not toxic to normal cell lines[11,19].
The molecular basis or mechanism for the growth inhibition activity of VES remains unclear, but it could be attributed to G1 cell cycle blockage[11,20], DNA synthesis arrest[4,7,21], increased expression of biologically active transforming growth factor-βs (TGF-βs) and their type II cell surface receptors[4,22], the induction of differentiation[23,24] and apoptosis[21,25,26]. VES is a potent inducer of apoptosis in human gastric cancer cells and it appears that at least two signaling pathways to trigger apoptosis may be involved. One of the previous studies in our laboratory have demonstrated that VES activates biologically active TGF-β and then TGF-β increases the kinase activity of c-Jun N-terminal kinase (JNK) followed by phosphorylation of c-Jun, and finally activated c-Jun triggers apoptosis in human gastric cancer cells[27]. The other shows that one of the death receptors, Fas plays an important role in VES-induced apoptosis, in that Fas activates Caspase-8 leading to a proteolytic cascade of Caspases through FADD[28].
It is well established that apoptosis or programmed cell death plays a pivotal role in the development and homeostasis of metazons by eliminating superfluous or unwanted cells[29-35]. Signals in response to stimulus-induced apoptosis or cellular stress affect the activity of transcription factors via several distant signal transduction pathways[36-38]. It is becoming clear that members of mitogen-activated protein kinase (MAPK) family have been shown to mediate almost all the cellular processes from gene expression to cell death[39-42]. In this study, we chose human gastric cancer cell line SGC-7901 as a model for VES-induced apoptosis. The roles of JNK, a member of MAPK family, were determined to further investigate the mechanism of VES-mediated growth inhibition of human gastric cancer cells.
VES was purchased from Sigma Co. Ltd. RPMI 1 640 media, LIPOFECTAMINE PLUSTM reagent and prestained protein marker were purchased from Gibco BRL, 4', 6-diamidine-2'-phenylindole dihydrochloride (DAPI) from Roche Diagnostics Co. Proteinase K from Merch Co. JNK and GAPDH rabbit polyclonal antibodies, dominant negative mutant construct of JNK were gifts from Dr. Bob G Sanders and Dr. Kimberly Kline (University of Texas, Austin, USA). Phospho-JNK mouse monoclonal and c-Jun (H79) rabbit polyclonal antibody were from Santa Cruz Biotechnologies.
Cell culture Human gastric cancer cell line SGC-7901 was maintained in RPMI 1 640 medium supplemented with 100 mL/L fetal calf serum (FCS), 100 kU/L penicillin, 100 mg/L streptomycin and 2 mmol/L L-glutamine under 50 mL/L CO2 in a humidified incubator at 37 °C. SGC-7901 cells were incubated for different periods in the presence of VES at 5, 10 and 20 mg/L (VES was dissolved in absolute ethanol and diluted in RPMI 1 640 complete condition medium correspondingly to a final concentration of VES and 1 mL/L ethanol). Both succinic acid and vitamin E dissolved in ethanol were used as vehicle controls and condition medium only was used as an untreated (UT) control.
DAPI staining and apoptotic evaluation Cells were treated with VES at 20 mg/L for 48 h, then harvested, washed with PBS and stained with 2 mg/L DAPI in 1 000 mL/L methanol for 30 min at 37 °C. Cells were viewed using a fluorescence microscope with ultraviolet (UV) excitation at 300-500 nm. Cells with nuclei containing clearly condensed chromatin or cells with fragmented nuclei were scored as apoptotic.
DNA fragmentation assay DNA fragmentation was determined by extraction of DNA followed by electrophoresis. In brief, cells were collected in an Eppendorf tube and washed twice with PBS. Cells were incubated for 1h at 37 °C in 0.5 mL of extraction buffer containing 10 mmol/L Tris·Cl (pH 8.0), 0.1 mol/L EDTA, 20 mg/L trypsin and 5 g/L SDS. The mixture was reincubated with 20 g/L proteinase K for 3 h at 50 °C. An equal volume of buffer saturated phenol was added and the extracted DNA was collected by centrifugation at 5 000 r/min for 15 min at room temperature. DNA was precipated by the addition of sodium acetate and absolute ethanol. DNA was dissolved in TE buffer and electrophoresed in 10 g/L agarose gel containing ethidium bromide and photographed under UV light.
Western blot analysis SGC-7901 cells treated with VES were harvested, washed with PBS and lyzed in lysis buffer (150 mmol/L NaCl, 1 mL/L NP-40, 5 mg/L sodium deoxycholate, 1 g/L SDS, 50 mmol/L Tris (pH 7.4), 1 mmol/L DTT, 0.5 mmol/L Na3VO4, 10 mmol/L phenylmethylsulfonyl fluoride (PMSF), 10 mg/L trypsin, 10 mg/L aprotinin and 5 mg/L leupeptin). Following the centrifugation of 12 000 g for 30 min at 4 °C, the amount of protein in the supernatant was determined using Biorad DC protein assay. Equal amounts of protein were separated on 100 g/L SDS-PAGE and transferred to different nitrocellulose filters (Gibco BRL, USA) overnight. Blocked with 50 g/L defatty milk, the individual filters were initially incubated with JNK, or with c-Jun, or with GAPDH rabbit polyclonal antibodies, or with phospho-JNK monoclonal antibody and then all were incubated again with horseradish peroxidase-conjugated IgG. Afterwards DAB was added to develop the filters.
Transient transfection SGC-7901 cells were washed twice with serum-free medium without antibiotics and incubated for 3 h in 2 mL of serum-free medium containing 30 μL of LIPOFECTAMINE reagent and 2 µg of dominant negative JNK or JNK vector. After 3 h, the cells were treated with VES.
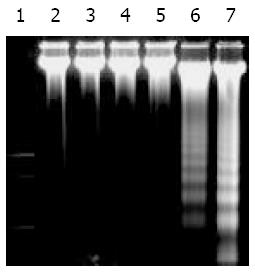
SGC-7901 cells were cultured for 48 h and collected, and DNA was extracted. Gel electrophoresis of DNA extracted from cells after exposure to UT control, succinate, vitamin E and VES is shown in Figure 1. Fragmentation of chromosomal DNA characterized as a DNA ladder was observed following exposure to VES at 10 and 20 mg/L. DNA ladder in 20 mg/L VES group was more clearly seen than that in 10 mg/L VES group. However, DNA ladder was not detected following treatment of UT control, succinate and vitamin E. These results suggested that VES induced human gastric cancer SGC-7901 cells to undergo apoptosis.
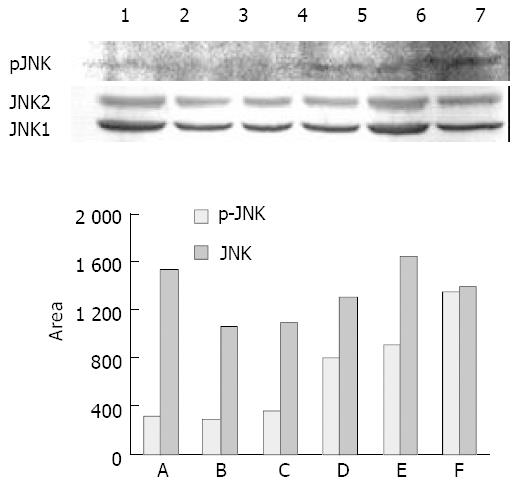
The expression of phospho-JNK (p-JNK) and JNK1/2 in the whole-cell lysates from UT control, succinate, vitamin E and VES-treated cells for 24 h was determined using Western blot analysis. The results revealed that VES increased the expression of p-JNK in an obvious dose-effect relationship. The levels of p-JNK protein in VES-stimulated cells at 5, 10 and 20 mg/L were increased by 2.5-, 2.8- and 4.2-fold over those in UT control-treated cells, respectively (Figure 2A, top panel; Figure 2B). The expression of JNK1/2 among different groups was not significantly different (Figure 2A, bottom panel; Figure 2B).
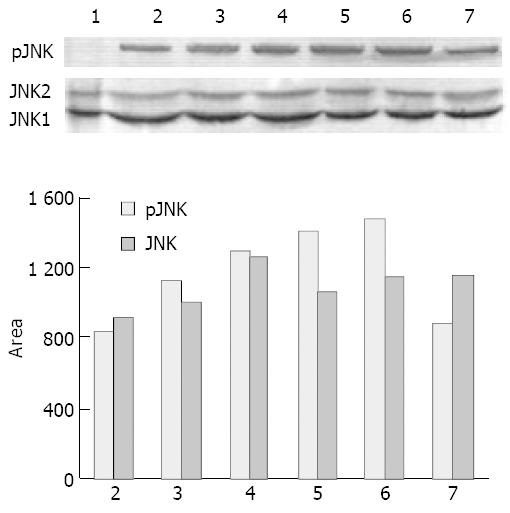
Since VES elevated the levels of p-JNK, we investigated whether VES might regulate the expression of p-JNK for 1.5, 3, 6, 12 and 24 h. VES at 20 mg/L induced a prolonged p-JNK expression starting at 1.5 h, peaking at 12 h and returning to the UT control level at 24 h after treatment (Figure 3A, top panel; Figure 3B). The levels of JNK1/2 were not increased by VES (Figure 3A, bottom panel; Figure 3B).
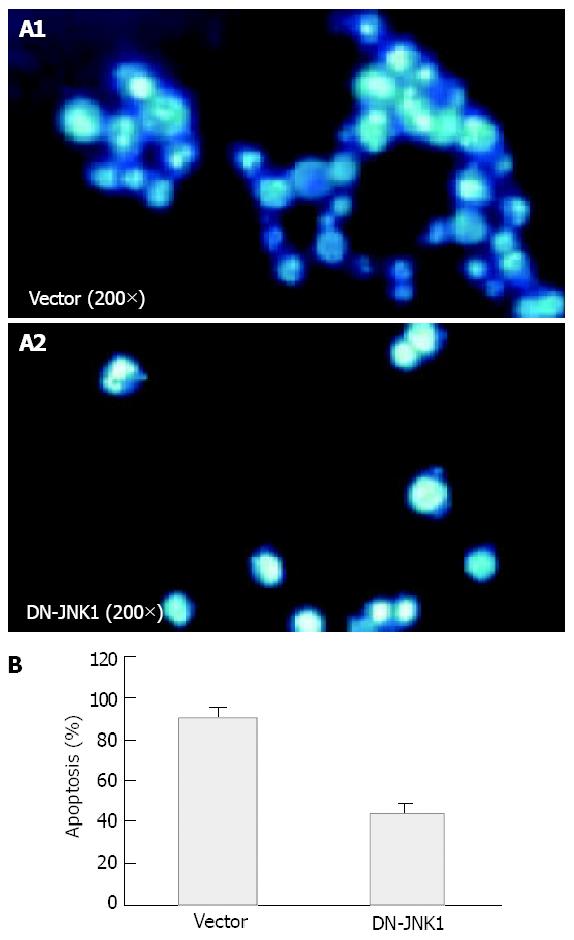
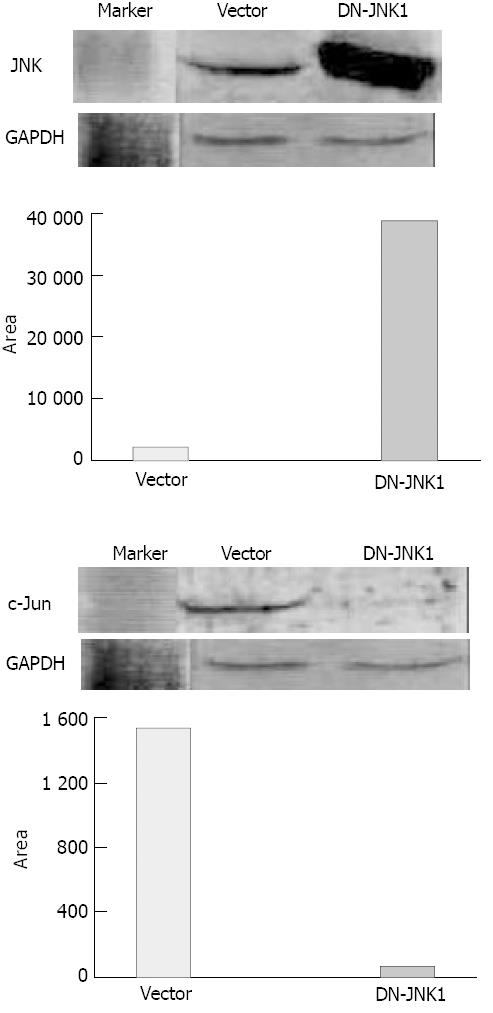
To further address the role of JNK signaling in VES-mediated apoptosis, studies were conducted to determine the effects of specific blockage of JNK with dominant negative mutants. SGC-7901 cells were transiently transfected with an expression construct containing dominant negative JNK (DN-JNK, pcDNA3-Flag-JNK, tyrosine 185 and threonine 183 required for phosphorylation activity were replaced with alanine and phenylalanine, repectively), followed by treatment of VES at 20 mg/L. For DAPI staining, SGC-7901 cells were transfected with DN-JNK and then treated with VES for 48 h. Then the cells were collected and stained with DAPI and photographed under a fluororescence microscope. DN-JNK reduced VES-induced apoptosis by 52% compared with the apoptotic rate in empty vector control cells (Figures 4A, 4B). In addition, DN-JNK significantly increased the levels of JNK by 18-fold (Figure 5, top panel), while decreasing the expression of c-Jun to a barely detectable level compared with those in the empty vector cells (Figure 5, middle panel). GAPDH protein levels served to verify lane loads (Figure 5, bottom panel).
Apoptosis has been found to be an active and physiological process characterized by a series of morphological and biochemical alterations, including condensation of cytoplasm, loss of plasma membrane microvilli, fragmentation of nucleus and extensive degradation of chromosomal DNA into oligomers of 180 bp by endonuclease[43,44]. Characteristic DNA ladder can be seen on agarose gel by electrophoresis. In this study, evident DNA ladder appeared in VES-treated SGC-7901 cells, especially at 20 mg/L VES. Therefore, VES can induce SGC-7901 cells to undergo apoptosis.
MAPKs are serine-threonine protein kinases that could be activated by diverse stimuli ranging from cytokines, growth factors, neurotransmitters, hormones, cellular stress and cell adherence[45-47]. MAPKs are evolutionarily conserved from yeast to human. MAPK activity is regulated through a three-tiered cascade composed of a MAPK kinase kinase (MKKK), a MAPK kinase (MKK/MEK) and a MAPK. Activated MAPKs could phosphorylate corresponding substrates, the majority of which are transcription factors[48]. Mammalian MAPKs can be subdivided into five groups, namely extracellular signal-regulated kinase (ERK) 1/2, c-Jun amino-terminal kinase (JNK), p38, ERK3/4 and ERK5.
JNK, also known as stress-activated protein kinase (SAPK), is phosphorylated by MKK4/7 activated by various MKKKs. Activated JNK in turn could phosphorylate transcription factors, c-Jun and ATF-2, which are components of the dimeric activating protein (AP)-1[49-51]. Here, we determined the expression of phospho-JNK and JNK in VES-stimulated SGC-7901 cells. The data showed that VES obviously increased the expression of p-JNK with a dose-effect relationship. The p-JNK levels were also elevated for a prolonged period after VES-treatment. VES induced activation of JNK beginning at 1.5 h after VES treatment and produced a sustain increase for 24 h with peak level at 12 h. The duration of JNK activation is critical in determining cell fate. Persistent activation of JNK has been shown to induce apoptosis. Thus, our results indicated a key role of JNK in VES-mediated apoptosis of human gastric cancer cells.
c-Jun transcription factor, a major target of JNK, belongs to an immediate early gene and could be rapidly and transiently induced in response to multiple extracellular stimuli[52-54]. Its expression can form homodimers or associate with other transcription factor partner, including members of Jun, Fos and ATF-2, to form heterodimeric complexes. Its activation through phosphorylation by JNK has been implicated in a variety of processes including embryomic developments, cellular transformation and initiation of apoptosis in response to various stresses[55-59]. JNK could phosphorylate c-Jun on serines 63 and 73 at the NH2-terminal activating sites. This results in increased stability of c-Jun and an increase in its transactivation potential and DNA binding affinity. Our previous studies showed that VES upregulated the expression of c-jun mRNA and protein in SGC-7901 cells[60]. In this study, transient transfection of dominant negative mutants of JNK (DN-JNK) blocked VES-triggered apoptosis by 52%. In addition, DN-JNK significantly increased the level of JNK, while decreased the expression of VES-induced c-Jun protein, indicating that JNK plays an important role in the regulation of c-Jun upstream.
Taken together, JNK is phosphorylated and activated in VES-induced apoptosis. JNK regulates the expression of c-Jun, a downstream transcription facor. All the data suggest that JNK plays a critical role in VES-induced apoptosis in human gastric cancer SGC-7901 cells. MAPK pathways are involved in a variety of responses affecting cell fate, such as cell proliferation and differentiation, adaptation to environment stress and apoptosis. None of the MAPK pathways including JNK is acting alone in cellular response, they are integrated with many other metabolic changes in the cells. Therefore, additional studies should provide insights into the interactions and significance among MAPK pathways.
We are grateful to Dr. Bob G Sanders and Dr. Kimberly Kline, University of Texas, Austin, USA, for giving us JNK and GAPDH antibodies and DN-JNK construct. We also thank Dr. Edgar J Love, University of Calgary, Canada, for critical reading of the manuscript.
Edited by Zhao M and Wang XL Proofread by Xu FM
| 1. | Wang YF, Li QF, Wang H, Mao Q, Wu CQ. Effects of vitamin E on experimental hepatic fibrosis in rats. Huaren Xiaohua Zazhi. 1998;6:207-209. |
| 2. | Jiang ZS, Gao Y. Biological feature of matrix metalloproteinase and its action in metastasis of liver cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:1403-1404. |
| 3. | Tso P, Lee T, DeMichele SJ. Randomized structured triglycerides increase lymphatic absorption of tocopherol and retinol compared with the equivalent physical mixture in a rat model of fat malabsorption. J Nutr. 2001;131:2157-2163. [PubMed] |
| 4. | Simmons-Menchaca M, Qian M, Yu W, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate inhibits DNA synthesis and enhances the production and secretion of biologically active transforming growth factor-beta by avian retrovirus-transformed lymphoid cells. Nutr Cancer. 1995;24:171-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Ottino P, Duncan JR. Effct of α-tocopheryl succinate on free radical and lipid peroxidation levels in BL6 melanoma cells. Free Radical Biol Med. 1997;22:1145-1151. [RCA] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Yu W, Sanders BG, Kline K. Modulation of murine EL-4 thymic lymphoma cell proliferation and cytokine production by vitamin E succinate. Nutr Cancer. 1996;25:137-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Yu W, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate inhibits EL4 thymic lymphoma cell growth by inducing apoptosis and DNA synthesis arrest. Nutr Cancer. 1997;27:92-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Fariss MW, Fortuna MB, Everett CK, Smith JD, Trent DF, Djuric Z. The selective antiproliferative effects of alpha-tocopheryl hemisuccinate and cholesteryl hemisuccinate on murine leukemia cells result from the action of the intact compounds. Cancer Res. 1994;54:3346-3351. [PubMed] |
| 9. | Israel K, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate inhibits the proliferation of human prostatic tumor cells with defective cell cycle/differentiation pathways. Nutr Cancer. 1995;24:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Zhang Y, Ni J, Messing EM, Chang E, Yang CR, Yeh S. Vitamin E succinate inhibits the function of androgen receptor and the expression of prostate-specific antigen in prostate cancer cells. Proc Natl Acad Sci USA. 2002;99:7408-7413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Kline K, Yu W, Sanders BG. Vitamin E: mechanisms of action as tumor cell growth inhibitors. Cancer and Nutrition. K.N. Prasad and W.C. Cole (Eds). IOS Press. 1998;37-53. |
| 12. | Turley JM, Ruscetti FW, Kim SJ, Fu T, Gou FV, Birchenall-Roberts MC. Vitamin E succinate inhibits proliferation of BT-20 human breast cancer cells: increased binding of cyclin A negatively regulates E2F transactivation activity. Cancer Res. 1997;57:2668-2675. [PubMed] |
| 13. | Kline K, Yu W, Sanders BG. Vitamin E: mechanisms of action as tumor cell growth inhibitors. J Nutr. 2001;131:161S-163S. [PubMed] |
| 14. | Liu B, Wu K, Zhao D. [Inhibition of human gastric carcinoma cell growth by vitamin E succinate]. Wei Sheng Yan Jiu. 2000;29:172-174. [PubMed] |
| 15. | Wu K, Guo J, Dan YJ, Liu BH. The effects of vitamin E succi-nate on apoptosis in human gastric cancer. Weisheng Dulixue Zazhi. 1999;13:84-90. |
| 16. | Schwartz J, Shklar G. The selective cytotoxic effect of caro-tenoids and α-tocopherol on human cancer cell lines in vitro. J Oral Maxillofac Surg. 1992;50:367-373. [RCA] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 74] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Wu K, Shan YJ, Zhao Y, Yu JW, Liu BH. Inhibitory effects of RRR-alpha-tocopheryl succinate on benzo(a)pyrene (B(a)P)-induced forestomach carcinogenesis in female mice. World J Gastroenterol. 2001;7:60-65. [PubMed] |
| 18. | Malafa MP, Neitzel LT. Vitamin E succinate promotes breast cancer tumor dormancy. J Surg Res. 2000;93:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 107] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Neuzil J, Weber T, Gellert N, Weber C. Selective cancer cell killing by alpha-tocopheryl succinate. Br J Cancer. 2001;84:87-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 190] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 20. | Kline K, Yu W, Zhao B, Israel K, Charpentier A, Simmons-Menchaca M, Sanders BG. Vitamin E Succinate: Mechanisms of action as tumor cell growth inhibitor. In: Nutrients in Cancer Prevention and Treatment. Prasad KN. Santamaria L and Williams RM (eds). Totowa.NY:Humana. 1995;39-56. [DOI] [Full Text] |
| 21. | Wu K, Zhao Y, Liu BH, Li Y, Liu F, Guo J, Yu WP. RRR-alpha-tocopheryl succinate inhibits human gastric cancer SGC-7901 cell growth by inducing apoptosis and DNA synthesis arrest. World J Gastroenterol. 2002;8:26-30. [PubMed] |
| 22. | Ariazi EA, Satomi Y, Ellis MJ, Haag JD, Shi W, Sattler CA, Gould MN. Activation of the transforming growth factor beta signaling pathway and induction of cytostasis and apoptosis in mammary carcinomas treated with the anticancer agent perillyl alcohol. Cancer Res. 1999;59:1917-1928. [PubMed] |
| 23. | Kim SJ, Bang OS, Lee YS, Kang SS. Production of inducible nitric oxide is required for monocytic differentiation of U937 cells induced by vitamin E-succinate. J Cell Sci. 1998;111:435-441. [PubMed] |
| 24. | You H, Yu W, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate induces MDA-MB-435 and MCF-7 human breast cancer cells to undergo differentiation. Cell Growth Differ. 2001;12:471-480. [PubMed] |
| 25. | Yu W, Israel K, Liao QY, Aldaz CM, Sanders BG, Kline K. Vitamin E succinate (VES) induces Fas sensitivity in human breast cancer cells: role for Mr 43,000 Fas in VES-triggered apoptosis. Cancer Res. 1999;59:953-961. [PubMed] |
| 26. | Neuzil J, Weber T, Schröder A, Lu M, Ostermann G, Gellert N, Mayne GC, Olejnicka B, Nègre-Salvayre A, Stícha M. Induction of cancer cell apoptosis by alpha-tocopheryl succinate: molecular pathways and structural requirements. FASEB J. 2001;15:403-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 219] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 27. | Wu K, Liu BH, Zhao DY, Zhao Y. Effect of vitamin E succinate on expression of TGF-beta1, c-Jun and JNK1 in human gastric cancer SGC-7901 cells. World J Gastroenterol. 2001;7:83-87. [PubMed] |
| 28. | Wu K, Li Y, Zhao Y, Shan YJ, Xia W, Yu WP, Zhao L. Roles of Fas signaling pathway in vitamin E succinate-induced apoptosis in human gastric cancer SGC-7901 cells. World J Gastroenterol. 2002;8:982-986. [PubMed] |
| 29. | Liu HF, Liu WW, Fang DC. Effect of combined anti Fas mAb and IFN-γ on the induction of apoptosis in human gastric 5 2 carcinoma cell line SGC-7901. Shijie Huaren Xiaohua Zazhi. 2000;8:1361-1364. |
| 30. | Yang JQ, Yang LY, Zhu HC. Mitomycin Cinduced apoptosis of human hepatoma cell. Shijie Huaren Xiaohua Zazhi. 2001;9:268-272. |
| 31. | Sun ZX, Ma QW, Zhao TD, Wei YL, Wang GS, Li JS. Apoptosis induced by norcantharidin in human tumor cells. World J Gastroenterol. 2000;6:263-265. [PubMed] |
| 32. | Peng ZH, Xing TH, Qiu GQ, Tang HM. Relationship between Fas/FasL expression and apoptosis of colon adenocarcinoma cell lines. World J Gastroenterol. 2001;7:88-92. [PubMed] |
| 33. | Xu AG, Li SG, Liu JH, Gan AH. Function of apoptosis and expression of the proteins Bcl-2, p53 and C-myc in the development of gastric cancer. World J Gastroenterol. 2001;7:403-406. [PubMed] |
| 34. | Zhao Y, Wu K. Cell death molecule Fas/CD95 and apoptosis. Aibian Jibian Tubian. 2001;13:55-58. |
| 35. | Yan J, Xu YH. Tributyrin inhibits human gastric cancer SGC-7901 cell growth by inducing apoptosis and DNA synthesis arrest. World J Gastroenterol. 2003;9:660-664. [PubMed] |
| 36. | Shen YF, Zhuang H, Shen JW, Chen SB. Cell apoptosis and neoplasms. Shijie Huaren Xiaohua Zazhi. 1999;7:267-268. |
| 37. | Liang WJ, Zhang WD. Signal conducting mechanism of tumor necrosis factor inducing apoptosis. Shijie Huaren Xiaohua Zazhi. 2000;8:329-331. |
| 38. | Sun BH, Zhao XP, Wang BJ, Yang DL, Hao LJ. FADD and TRADD expression and apoptosis in primary hepatocellular carcinoma. World J Gastroenterol. 2000;6:223-227. [PubMed] |
| 39. | Wu K, Zhao Y, Yu WP. Study on apoptosis. Guowai Yixue Yichuanxue Fence. 2001;24:134-138. |
| 40. | Xiong LJ, Zhu JF, Luo DD, Zen LL, Cai SQ. Effects of pentoxifylline on the hepatic content of TGF-beta1 and collagen in Schistosomiasis japonica mice with liver fibrosis. World J Gastroenterol. 2003;9:152-154. [PubMed] |
| 41. | Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3924] [Cited by in RCA: 4007] [Article Influence: 167.0] [Reference Citation Analysis (33)] |
| 42. | Bhalla US, Ram PT, Iyengar R. MAP kinase phosphatase as a locus of flexibility in a mitogen-activated protein kinase signaling network. Science. 2002;297:1018-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 450] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 43. | Ashkenazi A, Dixit VM. Apoptosis control by death and de-coy receptors. Curr Opin Cell Biol. 1999;11:255-260. [RCA] [DOI] [Full Text] [Cited by in Crossref: 975] [Cited by in RCA: 943] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 44. | Tao HQ, Zou SC. Effect of preoperative regional artery chemotherapy on proliferation and apoptosis of gastric carcinoma cells. World J Gastroenterol. 2002;8:451-454. [PubMed] |
| 45. | Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153-183. [RCA] [DOI] [Full Text] [Cited by in Crossref: 880] [Cited by in RCA: 861] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 46. | Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3062] [Cited by in RCA: 3307] [Article Influence: 143.8] [Reference Citation Analysis (0)] |
| 47. | Cowan KJ, Storey KB. Mitogen-activated protein kinases: new signaling pathways functioning in cellular responses to environmental stress. J Exp Biol. 2003;206:1107-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 447] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 48. | Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143-180. [PubMed] |
| 49. | Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci USA. 2003;100:2432-2437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 806] [Cited by in RCA: 830] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 50. | Clerk A, Kemp TJ, Harrison JG, Mullen AJ, Barton PJ, Sugden PH. Up-regulation of c-jun mRNA in cardiac myocytes requires the extracellular signal-regulated kinase cascade, but c-Jun N-terminal kinases are required for efficient up-regulation of c-Jun protein. Biochem J. 2002;368:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Mauro A, Ciccarelli C, De Cesaris P, Scoglio A, Bouché M, Molinaro M, Aquino A, Zani BM. PKCalpha-mediated ERK, JNK and p38 activation regulates the myogenic program in human rhabdomyosarcoma cells. J Cell Sci. 2002;115:3587-3599. [RCA] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 52. | Feng DY, Zheng H, Tan Y, Cheng RX. Effect of phosphorylation of MAPK and Stat3 and expression of c-fos and c-jun proteins on hepatocarcinogenesis and their clinical significance. World J Gastroenterol. 2001;7:33-36. [PubMed] |
| 53. | Zhu YH, Hu DR, Nie QH, Liu GD, Tan ZX. Study on activation and c-fos, c-jun expression of in vitro cultured human hepatic stellate cells. Shijie Huaren Xiaohua Zazhi. 2000;8:299-302. |
| 54. | Yuen MF, Wu PC, Lai VCH, Lau JYN, Lai CL. Expression of c-Myc, c-Fos, and c-jun in hepatocellular carcinoma. Cancer. 2001;91:106-112. [DOI] [Full Text] |
| 55. | Schroeter H, Spencer JPE, Rice-Evans C, Williams RJ. Fla-vonoids protect neurons from oxidized low-density-lipopro-tein-induced apoptosis involving c-Jun N-terminal kinase (JNK), c-Jun and caspase-3. Biochem J. 2001;358:547-557. [RCA] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 185] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 56. | Jiang LX, Fu XB, Sun TZ, Yang YH, Gu XM. Relationship be-tween oncogene c-jun activation and fibroblast growth factor receptor expression of ischemia reperfusion intestine in rats. Shijie Huaren Xiaohua Zazhi. 1999;7:498-500. |
| 57. | Fan M, Goodwin ME, Birrer MJ, Chambers TC. The c-Jun NH(2)-terminal protein kinase/AP-1 pathway is required for efficient apoptosis induced by vinblastine. Cancer Res. 2001;61:4450-4458. [PubMed] |
| 58. | Lei K, Nimnual A, Zong WX, Kennedy NJ, Flavell RA, Thomp-son CB, Bar-Sagi D, Davis RJ. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH2-terminal kinase. Mol Cell Biol. 2002;22:4929-4942. [RCA] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 400] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 59. | Qi X, Pramanik R, Wang J, Schultz RM, Maitra RK, Han J, DeLuca HF, Chen G. The p38 and JNK pathways cooperate to trans-activate vitamin D receptor via c-Jun/AP-1 and sensitize human breast cancer cells to vitamin D(3)-induced growth inhibition. J Biol Chem. 2002;277:25884-25892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 60. | Zhao Y, Wu K, Xia W, Shan YJ, Wu LJ, Yu WP. The effects of vitamin E succinate on the expression of c-jun gene and protein in human gastric cancer SGC-7901 cells. World J Gastroenterol. 2002;8:782-786. [PubMed] |