Published online Apr 1, 2004. doi: 10.3748/wjg.v10.i7.965
Revised: July 12, 2003
Accepted: August 16, 2003
Published online: April 1, 2004
AIM: To investigate the specific value of resistance index (RI) in color Doppler ultrasonography in the diagnosis of focal hepatic lesions.
METHODS: Eight hundred patients with 893 hepatic solid lesions were studied with color Doppler flow imaging (CDFI) and pulsed Doppler, including 644 malignant cases (596 primary malignant liver tumors, and 48 metastatic liver tumors), 156 benign cases. All were confirmed by operation and pathology.
RESULTS: The detection rate of arterial flow in malignant tumors was 92%, and 52% in benign lesions. Doppler spectrum analysis showed that the resistance index in primary malignant tumors was 0.75 ± 0.12, 0.73 ± 0.09 in metastatic tumors, and was below 0.6 in benign lesions. The difference was significant (P < 0.001). This difference was related with its histopathologic structure.
CONCLUSION: The arterial flow with RI ≥0.6 identified by CDFI within the liver lesion can be regarded as a criterion of malignant tumors, RI < 0.6 can be regarded as benign disorders. RI is useful in differential diagnosis of liver neoplasms.
- Citation: Wang Y, Wang WP, Ding H, Huang BJ, Mao F, Xu ZZ. Resistance index in differential diagnosis of liver lesions by color doppler ultrasonography. World J Gastroenterol 2004; 10(7): 965-967
- URL: https://www.wjgnet.com/1007-9327/full/v10/i7/965.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i7.965
Extensive use of high resolution ultrasonography has led to the detection of a large number of small focal lesions in general practice. However, differential diagnosis of benign and malignant liver lesions may be difficult, even with clinical, biochemical data, and imaging techniques[1,2]. Color Doppler flow imaging can provide information on blood flow, which is useful in the differential diagnosis of liver tumors[3]. Our study was to develop a standard protocol of color Doppler ultrasound for liver tumor vascularization and to assess resistance index (RI) in the differential diagnosis of liver lesions.
From 1992 to 2001, 800 patients with 893 hepatic solid lesions were studied (643 males, 157 females), their age ranged from 14-101 years (mean 50 years). The lesions included 644 malignancies (596 primary malignant liver tumors, and 48 metastatic liver tumors) and 156 benignancies. Primary malignant liver tumors included 564 hepatocellular carcinomas, 7 cholangiocarcinomas, and 12 mixed hepatocellular cholangiocarcinomas, 13 others. The benign lesions included 62 hepatic cavernous hemangiomas, 36 focal nodular hyperplasiae, 14 hepatic angiomyolipomas, 11 inflammatory pesudotumors of liver, 11 cirrhotic nodules, 5 hepatic tuberculosis, 3 hepatic adenomas, 3 liver abscesses, 2 liver lipomas, 9 others. The lesion size ranged from 6-180 mm in diameter. The histological distribution and tumor size are shown in Table 1.
| Histological type | Size (mm) | |||
| n (lesions) | < 30 | 30-60 | > 60 | |
| Primary malignant tumor | 596(651) | 232 | 255 | 164 |
| Metastatic tumor | 48(57) | 15 | 25 | 17 |
| Hemangioma | 62(86) | 25 | 33 | 28 |
| Fucal nodual hyperplasia | 36(36) | 19 | 16 | 1 |
| Inflammatory pseudotumor | 11(11) | 6 | 5 | |
| Angiomyolipoma | 14(17) | 3 | 10 | 4 |
| Cirrhotic nodules | 11(11) | 9 | 2 | |
| Tuberculosis | 5(6) | 4 | 2 | |
| Adenoma | 3(4) | 1 | 3 | |
| Abscess | 3(3) | 1 | 2 | |
| Lipoma | 2(2) | 1 | 1 | |
| Others | 9(9) | 2 | 5 | 2 |
| Total | 800(893) | 316 | 357 | 220 |
For ultrasonic evaluation of hepatic lesions, high-resolution ultrasonography equipment Acuson 128/XP10.USA.) with a 3.5MHz vector transducer was used. To minimize the splanchnic vasomotor influences, all patients were fasted overnight before sonographic evaluation. After the morphological characteristics of the lesions were assessed by B mode, Color Doppler ultrasound was used to determine the distribution, intra-and/or peritumoral vessels, and pulsed Doppler was used to point the interested lesions. Echogenicity and vascularity of the lesions were determined by using a magnification mode (Res) that not only provided simple geometric magnification but also improved the spatial resolution in the area of interest.
To visualize the blood flow, standard color Doppler sonography was used for each lesion, and pulsed Doppler was optimized to detect the low flow components by diminishing pulse repetition frequencies, usually down to 500Hz and adapting frequency filters. The color gain was manipulated until noise began to exceed the homogeneous single color background of color Doppler scans. Within the lesions, pulsed Doppler samples were assessed whenever possible on the basis of pulsatile flow. Vascularity could be arterial or venous. At least three measurements of resistance index (RI) of intratumoral and peritumoral arterial blood flow would be the last mean value. Only those showing the highest SPV values obtained with pulsed Doppler were taken into account. For statistical analysis, one-way ANOVA and χ2 test were used.
The 893 lesions were manifested as hyperechoic, hypoechoic or isoechoic masses with distinct or indistinct margins. In the real time ultrtasonography, intra-and/or perilesional blood flow signals presented as pulsed or continuous activity, slow blood flow showed a single color, and fast flow showed mixed color signals. The pulsating wave of arterial flow in malignant tumors, appeared as linear color signals or branching, whereas in benign tumors, a constant spectrum of venous flow was shown as dots or patches.
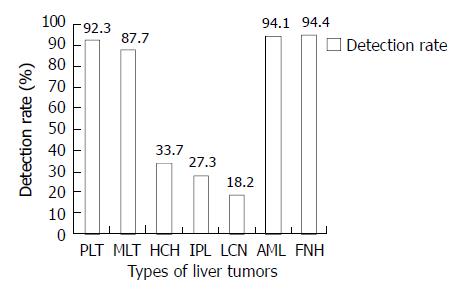
Intratumoral and peritumoral arterial flow signals were obtained in 92% of the malignant tumors, and in 52% of benign lesions (P < 0.01). The detection rate of intratumoral and peritumoral arterial flow was 92.3% in primary malignant liver tumors, 87.7% in metastatic liver tumors, and 33.7% in hemangiomas. The detection rates of intratumoral and peritumoral arterial flow in different lesions are shown in Figure 1. The detection rates of intratumoral and peritumoral arterial flow signals in malignant tumors, including primary or metastatic tumors were much higher than those in benign tumors, except angiomyolipomas and focal nodual hyperplasiae. Then RI was more helpful in differentiating the lesions having more arterial flow signals.
The average value of RI in primary malignant liver tumors was 0.75 ± 0.12 and 0.73 ± 0.09 in metastatic tumors (the difference was not statistically significant, and so was it in different histologic types). It was significantly higher than that in benign ones. RI of hemangiomas was 0.55 ± 0.08. The values of RI in different histological lesions are shown in Table 2. There were significant differences between malignant and benign tumors.
| Histopathological type | RI range | RI means |
| Primary malignant tumor | 0.34 - 1.0 | 0.75 ± 0.12b |
| Metastatic tumor | 0.57 - 1.0 | 0.73 ± 0.09b |
| Hemangioma | 0.45 - 0.77 | 0.55 ± 0.08 |
| Fucal nodual hyperplasia | 0.41 - 0.79 | 0.58 ± 0.10 |
| Angiomyolipoma | 0.38 - 0.71 | 0.52 ± 0.14 |
| Cirrhotic nodules | 0.57 - 0.59 | 0.58 ± 0.01 |
| Inflammatory pseudotumor | 0.49 - 0.55 | 0.54 ± 0.05 |
In our study we also found that the detection rates of intratumoral and/or peritumoral arterial flow signals and the value of RI in spectral analysis tended to be related to the tumor size, as arterial flow was easily detected in ≥ 2 cm lesions. The higher RI was mainly observed in smaller malignant tumors, but it decreased in larger tumors. The detection rates of arterial flow and the value of RI of vascular primary liver tumor (PLT) stratified by tumor size are shown in Table 3.
In our series, there were 50 lesions of PLT with no arterial flow signals. Most of them were hyperechoic masses, the size of 28 lesions was smaller than 30 mm. In 19 cases the position of the lesions was deeply located in the right posterial lobe. Eleven lesions near the diaphragm were interfered with the lung gas. Ten cases were in the left lateral lobe, 4 cases in the left medium lobe, 3 cases near the inferior vena cava (IVC), with interference of the heart beat. One case was necrosis, 2 cases were unable to control breath.
The type of blood flow signal (arterial or venous) and its distribution detected by color and pulsed Doppler is more helpful in differential diagnosis. Our study showed that the presence of both intra-and peritumoral arterial flow was strongly suggestive of malignancy, whereas the presence of intratumoral venous flow was remarkably suggestive of benignancy[4-7]. Intratumoral and peritumoral arterial flow signals were obtained in 92% of malignant tumors, but only in 52% of benign lesions. In the present study, with improvement of the equipment, we also found some arterial flow in some of the benign lesions, such as focal nodual hyperplasia (FNH) and angiomyolipoma (AML). How to differentiate them from malignant tumors remains a question. In our study, RI was more useful, combination of the type of signals and RI could significantly increase the accuracy of diagnosis. In 1997, Gonzalez-Anon et al[8] evaluated these aspects and the distribution of tumoral vessels, and concluded that the type of signals (arterial or venous) and its distribution detected by color and pulsed Doppler was more helpful than the assessment of quantitative spectral parameters obtained by pulsed Doppler. Some researchers attempted to characterize tumors by quantitative spectral criteria only, and found that systolic peak velocity (SPV) was above 70 cm/s in 12 hepatocarcinomas in their series, the velocities differed significantly from those found in metastases and hemangiomas. Numata et al[9] found that the mean of SPV in hepatocarcinomas was significantly higher than that of metastases, and hemangiomas. They also correlated the findings by Doppler angiography and hepatic pathology in both experimental animals and human beings, the high velocities of systolic peak was related with the presence of arterial venous shunts and the low resistant spectra were associated with vascular channels in the absence of muscle layer[10]. Since then, many attempts have been made to assess the usefulness of Doppler in the study of liver tumors[11]. Someda, et al[12] found that the arteries supplying HCCs had lower PI and higher PSV. Some investigators found a remarkable overlap between the spectral values of metastases and primary carcinomas. Kamalov et al[13] studied 128 lesions of primary and metastatic liver tumors, and found that the velocity and RI had no significance in differential diagnosis of tumors. In our experience, the value of SPV could usually be affected by the Doppler angle, but in most cases, the course of tumor vessels could not be determined. So the mean of SPV varied greatly, and high SPV usually presented in larger tumors, and was not significant in the differentiation of small lesions. RI (resistance index = (Vsp-Ved)/Vsp) presents the resistance of distal vessels. It is not influenced by the Doppler angle. In 1991, Xu et al and Wang et al studied the value of RI in differentiation of liver tumors, and concluded that RI > 0.5 was usually observed in malignant tumors, and RI < 0.5 was found in the hemangiomas[14,15]. In our further study, the statistical analysis of 800 patients also showed that RI in malignant tumors was significantly higher than that in benign tumors. The average value of RI in primary liver malignant tumors was 0.75 ± 0.12, and 0.73 ± 0.09 in metastatic tumors. They were much higher than that of benignancies. Pulsed Doppler spectrum analysis showed that the lesions without any signal during diastole or with diastolic reversal spectrum were all malignant. The mean value of RI in hemangiomas was 0.55 ± 0.08. Furthermore, the larger the tumor size was, the more the arterial flow could be visualized. The detection rates of intratumoral and/or peritumoral arterial flow signals and the value of RI in spectral analysis tended to be related to the tumor size. High RI was mainly observed in smaller malignant nodules, but it decreased in larger malignant tumors. It is conceivable that several factors are involved such as histological pattern, pseudocapsular growth type, and absence of necrotic areas. These would contribute to the increase of vascular impedance in small tumors. Some angioarchitectural features may also contribute to the explanation of the peculiar hemodynamic pattern observed. The nodule comprises exclusively arterial tumoral vessels, and hepatocytes are arranged in trabeculae of varying thickness that may compress the interposed vascular space, producing multiple “stenoses”. Furthermore, the presence of pesudocapsules and cirrhotic parenchymas that usually surround HCCs might affect the venous outflow by compression of peritumoral portal branches. But in larger tumors (diameter > 6 cm) with formation of A-V shunt and destruction of pseudocapsules, the value of RI tended to be lower.
In our series, combination of color Doppler flow imaging and RI was more helpful in differentiating malignant from benign tumors. Some of FNH, AML usually had more arterial flow just like malignant tumors, but they showed peculiar angioarchitectural features. FNH was characterized pathologically by cholangiolar proliferation associated with hyperplastic hepatocytes, blood vessels and fibrosis. AML was characterized pathologically by vessels with a thick muscle layer, showing more arterial flow signals like “blood ball”. They often showed high peak velocity and low impedance, RI was usually < 0.6. In the other aspect, RI was more useful to differentiate some benign lesions with less blood flow such as inflammatory pseudotumors of liver and cirrhotic nodules. Inflammatory pseudotumors of liver had no blood flow or less peritumor flow signals with RI < 0.6. Cirrhotic nodules usually had venous intratumoral flow or less peritumor flow with RI < 0.6.
The factors affecting RI in the diagnosis of liver lesions include: (1) sensitivity of the equipment. The difference in findings of flow is presumably due to the sensitivity of the equipment used for Doppler frequency shifts. Thus even lower and finer blood flows can probably be visualized if newer instruments are developed. (2) Management of the equipment. To visualize blood flow, pulsed Doppler is optimized to detect even low flow components by deminishing pulse repetition frequencies, usually down to 500Hz and adapting frequency filters. (3) Color imaging of blood flow is insufficient when the tumor is located deep within in the liver. (4) Tumor size. It is difficult to visualize when tumor size is less than 2 cm with deep location or near the diaphragm. (5) Cirrhotic parenchyma of the liver usually causes acoustic attenuation. (6) The angle of Doppler can affect the sensitivity of CDFI.
We should notice that the lesion without blood flow signals is presumably due to the angioarchitectural features or the above factors. In our series, most of malignant tumors without blood flow were usually smaller than 3 cm, and deeply located in the right posterial lobe of the liver or near the diaphragm, or located in the left lateral lobe with interference of the lung gas and the heart beating.
In conclusion, the type of flow signals (arterial and/or venous) and its distribution in CDFI and pulsed Doppler are helpful in differentiating benign from malignant lesions. The presence of intratumoral venous flow is strongly suggestive of benign tumors. When intra-and/or peritumoral arterial blood flow is found, RI < 0.6 would strongly suggest a benign tumor. Simultaneous occurrence of both intra-and peritumoral arterial flow and RI ≥ 0.6 would strongly suggest malignancies. So that combined studies of the type of intra-and peritumoral flow signals in CDFI and the parameter of RI would be more helpful in differential diagnosis of benign and malignant liver tumors.
Edited by Wang XL and Xu FM
| 1. | Furuse J, Iwasaki M, Yoshino M, Konishi M, Kawano N, Kinoshita T, Ryu M. Evaluation of blood flow signal in small hepatic nodules by color Doppler ultrasonography. Jpn J Clin Oncol. 1996;26:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Perkins AB, Imam K, Smith WJ, Cronan JJ. Color and power Doppler sonography of liver hemangiomas: a dream unfulfilled? J Clin Ultrasound. 2000;28:159-165. [PubMed] [DOI] [Full Text] |
| 3. | Tanaka S, Kitamura T, Fujita M, Nakanishi K, Okuda S. Color Doppler flow imaging of liver tumors. AJR Am J Roentgenol. 1990;154:509-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 155] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Ralls PW, Johnson MB, Lee KP, Radin DR, Halls J. Color Doppler sonography in hepatocellular carcinoma. Am J Physiol Imaging. 1991;6:57-61. [PubMed] |
| 5. | Srivastava DN, Mahajan A, Berry M, Sharma MP. Colour Doppler flow imaging of focal hepatic lesions. Australas Radiol. 2000;44:285-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Tang J. [Color Doppler flow imaging and duplex Doppler in the examination of primary liver cancer]. Zhonghua Zhongliu Zazhi. 1992;14:138-140. [PubMed] |
| 7. | Nino-Murcia M, Ralls PW, Jeffrey RB, Johnson M. Color flow Doppler characterization of focal hepatic lesions. AJR Am J Roentgenol. 1992;159:1195-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 50] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | González-Añón M, Cervera-Deval J, García-Vila JH, Bordón-Ferré F, Ambit-Capdevila S, Piqueras-Olmeda R, Jornet-Fayos J, Gil-Sánchez S, Marco-Domenech SF, Cortés-Vizcaíno V. Characterization of solid liver lesions with color and pulsed Doppler imaging. Abdom Imaging. 1999;24:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Numata K, Tanaka K, Kiba T, Morimoto M, Arata S, Kondo M, Sekihara H. Use of hepatic tumor index on color Doppler sonography for differentiating large hepatic tumors. AJR Am J Roentgenol. 1997;168:991-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Numata K, Tanaka K, Mitsui K, Morimoto M, Inoue S, Yonezawa H. Flow characteristics of hepatic tumors at color Doppler sonography: correlation with arteriographic findings. AJR Am J Roentgenol. 1993;160:515-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Uggowitzer M, Kugler C, Machan L, Gröll R, Stauber R, Mischinger HJ, Ratschek M, Fotter R. Power Doppler imaging and evaluation of the resistive index in focal nodular hyperplasia of the liver. Abdom Imaging. 1997;22:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Someda H, Moriyasu F, Hamato N, Fujimoto M, Okuma M. Change in hepatic arterial hemodynamics induced by hepatocellular carcinoma detected with Doppler sonography. J Clin Ultrasound. 1997;25:359-365. [PubMed] [DOI] [Full Text] |
| 13. | Kamalov IR, Sandrikov VA, Gautier SV, Tsirulnikova OM, Skipenko OG. The significance of colour velocity and spectral Doppler ultrasound in the differentiation of liver tumours. Eur J Ultrasound. 1998;7:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Wang WP, Xu ZZ, Shen SC. [Combined color Doppler and pulsed Doppler in the diagnosis of small hepatocellular carcinomas]. Zhonghua Wai Ke Za Zhi. 1994;32:474-476. [PubMed] |
| 15. | Xu ZZ, Wang WP. Application of ultrasonic Doppler in the diag-nosis of the hepatic solid space-occupying lesions. Zhonghua Wuli Yixue Zazhi. 1991;13:65-69. |