Published online Apr 1, 2004. doi: 10.3748/wjg.v10.i7.959
Revised: January 3, 2004
Accepted: January 8, 2004
Published online: April 1, 2004
AIM: To investigate the effects of hepatitis B virus x gene and its protein product HBxAg on apoptosis in hepatocyte line HL-7702.
METHODS: The reconstituted plasmid pcDNA3-x was established through recombination DNA technique; pcDNA3-X was transfected into HL-7702 cells by lipid-mediated trasfection. Positive clones were screened by G418, and HL-7702/HBx cells were analysed by the RT-PCR to confirm the steady expression of X gene in HL-7702 cells. The apoptosis rate in HL-7702 cells was determined by flow cytometry, TUNEL technology, electronic microscope. At the mean time, pcDNA3-X was transfected transiently into HL-7702 cells, and total RNA from HL-7702 cells was extracted 24, 48, 72, 96 and 120 h after the transient transfection, and semi-quantitative analysis was performed by RT-PCR to detect the expression of HBV X gene. Furthermore, apoptosis rate in HL-7702 cells was determined by flow cytometry analysis at the different times.
RESULTS: RT-PCR analysis showed that HBV X gene could be expressed stably in HL-7702 cells. Both flow cytometry and TUNEL technology revealed that the apoptosis rates of HL-7702/HBx cells were much higher than those of HL-7702/ pcDNA3 and HL-7702 cells. Furthermore, the apoptotic phenomena and apoptotic body were observed in HL-7702/ HBx cells under electronic microscope, but not in HL-7702/ pcDNA3 and HL-7702 cells. In the experiment of transient transfection, RT-PCR reveals that X gene was expressed most at 72 h after transfection; and the apoptosis rate reached the highest at the same time. After that, the apoptosis rate was reduced with the decrease of the X gene expression.
CONCLUSION: HBV X gene and X protein can promote the apoptosis in hepatocyte. And there exist a quantity-effect relationship between the X gene expression and apoptosis rate in hepatocyte.
- Citation: Chen HY, Tang NH, Li XJ, Zhang SJ, Chen ZX, Wang XZ. Transfection and expression of hepatitis B virus x gene and its effect on apoptosis in HL-7702 cells. World J Gastroenterol 2004; 10(7): 959-964
- URL: https://www.wjgnet.com/1007-9327/full/v10/i7/959.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i7.959
Chronic Hepatitis B virus (HBV) infection is associated with a high incidence of liver disease, including hepatitis and hepatocellular carcinoma (HCC)[1-3]. Based on epidemiologic studies involving chronic HBV infection, it is estimated that the relative risk of developing HCC for HBV carriers may be 100- to 200-fold higher than that for non-carriers[4].
HBV genome has four open reading frames (ORFs), including envelope genes coding region (pre-s1, pre-s2 and s gene coding region), precore (pc) gene and core (c) gene coding region, polymerase (p) gene coding region, X gene coding region. X gene is the smallest open reading frame of HBV. X gene codes for a 16.5-ku protein (X protein, HBx), which is made of 154 amino acids. HBx is a multifunctional viral regulator that modulates transcription, cell responses to genotoxic stress, protein degradation, and signaling pathways[5]. Besides, it may affect DNA repair, cell cycle control, and apoptosis[6-9].
To explore the effection of HBV X gene and its protein product on apoptosis, we transfected X gene into hepatocyte line HL-7702, and observed its role on cell apoptosis by flow cytometry, TUNEL technology and electronic microscope.
Restriction end onucleases, T4 DNA ligase, reverse transcription system, G418 and Transfast transfection reagent were obtained from Promega Biotech (USA). Vector PUCmT was purchased from Shanhai Sangon Co. (China). RNA isolation kit was from Jingmei Biotech Co. (China). TUNEL reagent was obtained from Beijing Zhongshan Co. (China). The plasmid PcDNA3 was from Invitrogen Co. (USA).
The HBV X DNA fragment was amplified by polymerase chain reaction (PCR) using the HBV carrier’s serum as a template. The sequences of primers for the PCR were as follows: 5’-ATGCAAGCTTATGGCTGCTAGGCTGTACTG-3’and5’-TGCGAATTCTTAGGCAGAGGTGAAAAAGTTG-3’. The PCR was carried out as follows: pre-denaturation at 95 °C for 5 min, 30 amplification cycles (denaturation at 94 °C for 45 s, annealing at 61 °C for 35 s, and extension at 72 °C for l min). After the PCR product was added an A-tailing, it was connected with vector pUCmT to construct the middle vector pUCmT-X. According to the sequencing result of pUCmT, the positive clone was selected to extract plasmid. Then the plasmid was digested with EcoR I and Hind III and ligated into pcDNA3 to establish the reconstituted plasmid pcDNA3-X, which was confirmed by restriction end onucleases digestion and direct DNA sequencing.
Cell culture and DNA transfection HL-7702 cells were cultured in RPMI 1640 supplemented with 200 mL/L FBS. The cells in logarithmic glowth were separately transfected with pcDNA3-X and pcDNA3 plasmids, by lipid-mediated trasfection using Transfast transfection reagent with a total of 2 μg of DNA per well in a 6-well plate. G418 was added to select the resistant clones after 48 h. After 2-wk selection, positive clones that were named HL-7702/HBx and HL-7702/ pcDNA3 were isolated and further expanded.
RT-PCR analysis Total RNA was extracted separately from the cells of each group (HL-7702/HBx, HL-7702/pcDNA3, HL-7702) with RNA isolatione kit, and RT-PCR was carried out. Using 5 μL of RT product as template, X gene andβ-actin were amplified together by PCR. The sequence of X gene primers for RT-PCR were as described above. The primers of β-actin were: 5-’GGCATCGTGATGGACTCCG-3’ and 5’-GCTGGAAGGTGGACAGCGA-3’. PCR was carried out as follows: Pre-denaturation for 5 min at 95 °C; 32 amplification cycles (denaturation at 94 °C for 35 s, annealing at 65 °C for 35 s, and extension at 72 °C for l min).
Flow cytometric analysis The cells of three groups were harvested with trypsin, resuspended in PBS (1 × 106 cells/mL), and analyzed for cell apoptosis rate by flow cytometry.
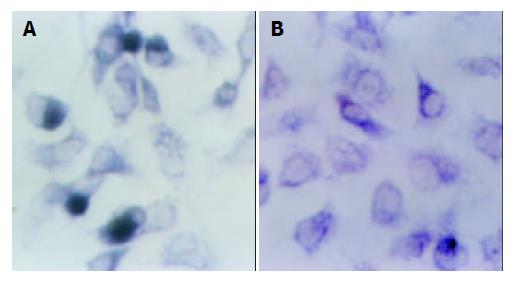
TUNEL technology The cells of three groups were separately seeded into the wells of 96-well plate. When cells nearly full confluent, they were fixed for 1 h at 37 °C. Tunel assay was performed following the manufacturer’s instructions. Finally, the cells were analyzed under a microscope. The apoptosis cells which had blue-black nucleus were counted among 3 000 cells.
Electronic microscope observation A total of 1 × 106 cells of HL-7702/HBx or HL-7702/PcDNA3 or HL-7702 were digested, centrifuged, fixed and analyzed for cell apoptosis under an electronic microscope.
Instantaneous transfection HL-7702 cells were cultured in RPMI 1640 supplemented with 200 mL/L FBS. The cells in logarithmic glowth were separately transfected with pcDNA3-X and pcDNA3 plasmids with Transfast transfection reagent with a total of 2 μg of DNA per well in a 6-well plate.
RT-PCR semi-quantitative analysis At the time of 24, 48, 72, 96, 120 h of post-transfection, RNA from the cells of 3 groups were separately extracted and transcribed reversely to cDNA. The RT reaction contained 2.5 μg RNA, 2 μL of 10 × buffer, 4 μL of MgCl2, 2 μL of dNTP (10 mmol/L), 0.5 μL of RNasin, 0.6 μL AMV (24 U/μL). A 20 μL total RT reaction volume was obtained by adding sterile water, and RT was carried out at 42 °C for 1 h and 95 °C for 5 min. Then using 5 μL RT product as template, X gene and β-actin were amplified together by PCR as described above. The semi-quantitative analysis for the expression of HBV X gene was performed through the luminosity contrast between X gene andβ-actin at the different time under ultraviolet ray. This experiment was repeated under the same conditions 3 times.
Flow cytometric analysis At the 5 different times, the cells of 3 groups were harvested by trypsinization, resuspended in PBS (1 × 106 cells/mL), and analyzed for cell apoptosis by flow cytometry.
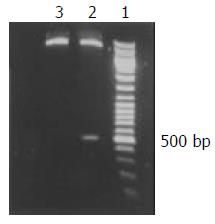
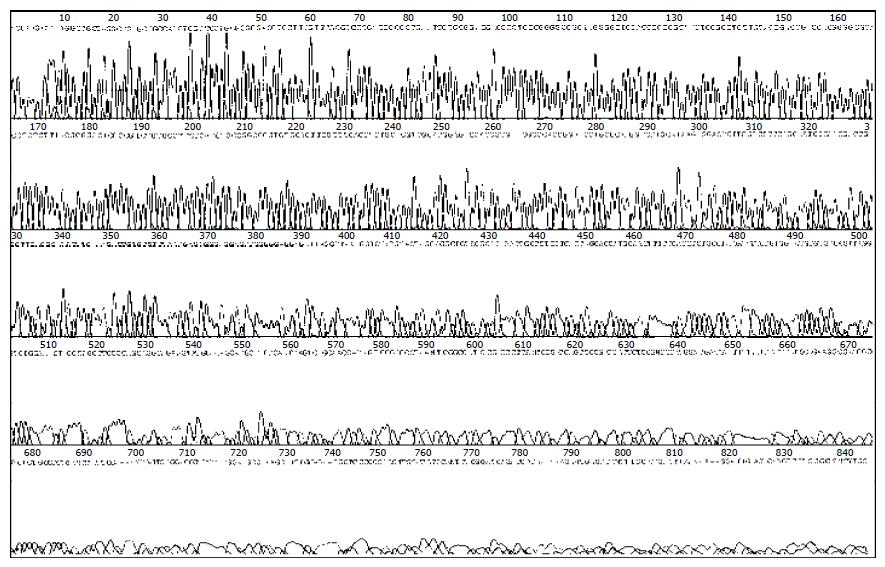
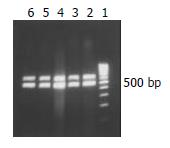
A 465 bp HBV X gene was amplificated from HBV genomic DNA and subcloned into the expression vector pcDNA3, with which the pcDNA3-X was constructed. The sequence of X gene in the plasmid was coincident with that reported before as identified by restriction endonucleases digestion (Figure 1) and confined by DNA direct sequencing (Figure 2).
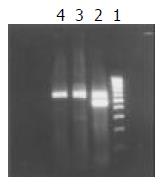
After pcDNA3-X plasmids were transfected into HL-7702 cells, the cells were isolated by G418 selection. HBV X mRNA was detected in HL-7702/HBx cells by RT-PCR which suggested that pcDNA3-X plasmids were expressed steadily in eukaryotic cells (Figure 3).
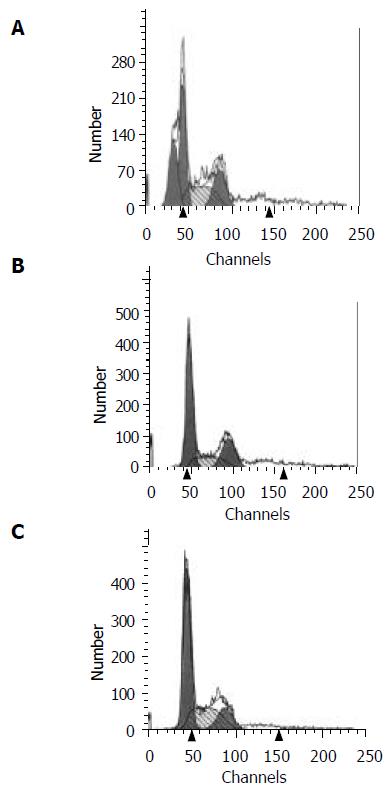
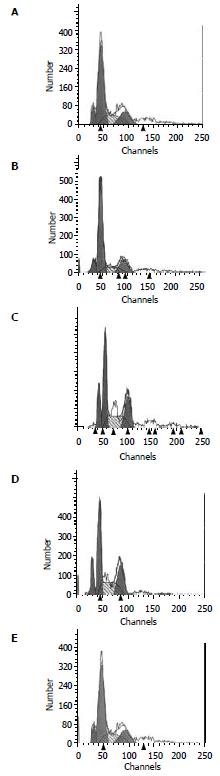
Flow cytometric analysis The apoptosis rate of HL-7702/HBx cells was 23%, while no apoptosis of cells was found in the HL-7702/pcDNA3 and HL-7702 cells (Figure 4).
TUNEL analysis Of 3000 HL-7702/HBx cells counted, 589 cells underwent apoptotic cell death, while in the HL-7702/ pcDNA3 and HL-7702 cells only 36 and 29 cells underwent apoptotic cell death, respectively. Apoptosis was significantly increased in HL-7702/HBx cells compared with the other two groups (using SAS software, χ2 test: P1, P2 < 0.01, Figure 5).
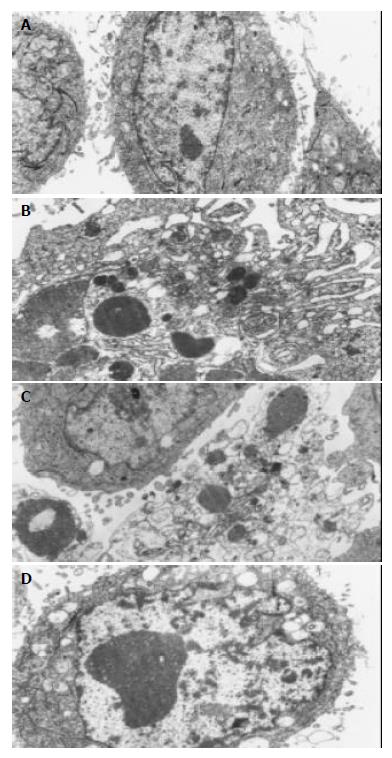
Electronic microscope analysis Under electronic microscope, typical apoptosis phenomena were found in HL-7702/HBx cells: the outward appearance of cells look like plum blossom; the membrane existed entirely; stainable feature concentrate into blocks and break down; the decreased density of cytoplasm; endoplasmic reticulum looked like bubbles (Figure 6B). And apoptotic body (Figure 6C), assemblage of heterochromatin and edema of mitochondria (Figure 6D) were found in some apoptotic cells, While none of apoptotic phenomena were observed in the HL-7702/PcDNA3 and HL-7702 cells (Figure 6A).
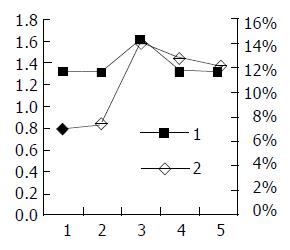
RT-PCR semi-quantitative analysis The result of RT-PCR can be seen in Figure 7. The luminosity contrasts between X gene and β-actin at the different times is described in Table 1. The results suggest that HBV X gene was expressed the first day after transfection, and most strongly expressed 72 h after transfection. After that, the expression of HBV X gene decreased with time.
| The time aftertransfection(h) | Firsttime | Secondtime | Thirdtime | Average |
| 24 | 1.3234 | 1.2823 | 1.3333 | 1.3130 |
| 48 | 1.2396 | 1.3433 | 1.3568 | 1.3132 |
| 72 | 1.5884 | 1.6212 | 1.6345 | 1.6147 |
| 96 | 1.2814 | 1.3612 | 1.3245 | 1.3224 |
| 120 | 1.3224 | 1.3041 | 1.3012 | 1.3092 |
Flow cytometric analysis As shown in Figure 8A-E, the apoptosis phenomena were observed the first day after transfection, and reach the highest at 72 h, then dropped again.
The link between apoptosis rate and X gene expression A two-axis broken line graph was drawn in order to determine the association between HBV X gene expression and cell apoptosis rate (Figure 9).
The HBx protein is a small polypeptide encoded by mammalian hepadnaviruses that is essential for viral infectivity and is thought to play a role in development of hepatocellular carcinoma during chronic hepatitis B virus infection[10]. It is known to be a transactivator of transcriptional elements that regulates the expression of a variety of genes associated with the growth, differentiation, survival and the apoptosis of cells. However, the role of the hepatitis B virus protein HBx in liver cell proliferation and apoptosis remains controversial. A number of studies have revealed that HBx protein exerts dual activity on cell apoptosis[11].
On one hand, HBx protein can block apoptosis through the following ways: (1) upregulates the activity of NF-kappa B[12] and activates NF-kappa B to translocate into nuclei of hepatocellular carcinoma cells[13,14]; (2) upregulates the expression of FasL in hepatoma cells, which contribute to tumor escape from immune surveillance of the body[15,16]; (3) abrogates Bcl-2-mediated protection against Fas apoptosis in the liver[17];(4) decreases the expression of Bid[18]; (5) inhibits caspase 3 activity[19]; (6) inhibits transforming growth factor beta-induced apoptosis through the activation of phosphatidylinositol 3-kinase pathway[20].
On the other hand, increasing evidence here suggested that HBx may exert a proapoptotic effect on hepatocytes and in the liver of HBx transgenic mice[9,21-31]. HBx protein has been shown to induce or sensitize cells to apoptotic killing by proapoptotic stimuli, such as etoposide and tumor necrosis factorα[24,25,32,33]. Terradillos et al[23] reported that HBx induced apoptosis in a p53-independent manner, but Chirillo et al[26] reported p53-dependent apoptotic killing of cells by HBx. However, a recent study could not substantiate that HBx has any detectable effect on p53 activity or location[34]. In addition, HBx can also lead to several activities, including stimulation of Ras, activition of Src kinases, transcriptional activation of myc genes, constitutive activation of JNKs, and loss of cell cycle checkpoint controls, thereby leading to cell apoptosis[9]. Moreover, HBx could interact with mitochondria, cause loss of mitochondrial membrane potential[35] and mitochondrial aggregation at the nuclear periphery[31], leading to cell death.
The dual activity of HBx protein on cell death suggests that the expression of HBx gene and its physiological role in viral pathogenesis are not simple and might be tightly regulated[11]. It seems that HBx may have evolved strategies to block and/or induce apoptosis depending on the cellular environment[24] and infection stage. Thus, HBx protein may play an opposite role in the different cellular environments and different infection stages.
Here, we constructed the HL-7702/HBx cells which could espress HBV X gene steadily and found that X gene able to promote cell apoptosis. In addition, a dose-dependent apoptotic function of HBx was demonstrated in transient transfections of liver cell lines. There existed quantity-effect relationship between the X gene expression and apoptosis rate, suggesting that the more X gene expression, the higher apoptosis rate in liver cells.
However, the precise role of HBx-mediated apoptosis is still unkown. It has been proposed that induction of apoptosis by HBx can actually facilitate propagation of viral infection by permitting efficient particle release from cells while minimizing the antiviral inflammatory response[9]. Alternatively, it is also possible that stimulation of apoptosis by HBx might promote release of hepatocyte growth factors, enhancing regenerative responses and increasing the level of uninfected hepatocytes for new infection, ultimately leading to liver cell transformation[9,12,24]. And the apoptotic effects of HBx by naturally occurring mutations might render the hepatocytes susceptible to uncontrolled growth and contribute to multistep hepatocarcinogenesis associated with HBV-infection. Further study is required to explore the mechanisms and roles of HBx-mediated apoptosis.
Edited by Gupta MK and Xu FM
| 1. | Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1202] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 2. | Chisari FV. Rous-Whipple Award Lecture. Viruses, immunity, and cancer: lessons from hepatitis B. Am J Pathol. 2000;156:1117-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 229] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 3. | Montalto G, Cervello M, Giannitrapani L, Dantona F, Terranova A, Castagnetta LA. Epidemiology, risk factors, and natural history of hepatocellular carcinoma. Ann N Y Acad Sci. 2002;963:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 207] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Xiong J, Yao YC, Zi XY, Li JX, Wang XM, Ye XT, Zhao SM, Yan YB, Yu HY, Hu YP. Expression of hepatitis B virus X protein in transgenic mice. World J Gastroenterol. 2003;9:112-116. [PubMed] |
| 5. | Murakami S. Hepatitis B virus X protein: a multifunctional viral regulator. J Gastroenterol. 2001;36:651-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 260] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | Koike K, Moriya K, Yotsuyanagi H, Iino S, Kurokawa K. Induction of cell cycle progression by hepatitis B virus HBx gene expression in quiescent mouse fibroblasts. J Clin Invest. 1994;94:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 106] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Benn J, Schneider RJ. Hepatitis B virus HBx protein deregulates cell cycle checkpoint controls. Proc Natl Acad Sci U S A. 1995;92:11215-11219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 236] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Shih WL, Kuo ML, Chuang SE, Cheng AL, Doong SL. Hepatitis B virus X protein inhibits transforming growth factor-beta -induced apoptosis through the activation of phosphatidylinositol 3-kinase pathway. J Biol Chem. 2000;275:25858-25864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 159] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Su F, Theodosis CN, Schneider RJ. Role of NF-kappaB and myc proteins in apoptosis induced by hepatitis B virus HBx protein. J Virol. 2001;75:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Wang XZ, Jiang XR, Chen XC, Chen ZX, Li D, Lin JY, Tao QM. Seek protein which can interact with hepatitis B virus X protein from human liver cDNA library by yeast two-hybrid system. World J Gastroenterol. 2002;8:95-98. [PubMed] |
| 11. | Jin YM, Yun C, Park C, Wang HJ, Cho H. Expression of hepatitis B virus X protein is closely correlated with the high periportal inflammatory activity of liver diseases. J Viral Hepat. 2001;8:322-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Su F, Schneider RJ. Hepatitis B virus HBx protein activates transcription factor NF-kappaB by acting on multiple cytoplasmic inhibitors of rel-related proteins. J Virol. 1996;70:4558-4566. [PubMed] |
| 13. | Guo SP, Wang WL, Zhai YQ, Zhao YL. Expression of nuclear factor-kappa B in hepatocellular carcinoma and its relation with the X protein of hepatitis B virus. World J Gastroenterol. 2001;7:340-344. [PubMed] |
| 14. | Madden CR, Slagle BL. Stimulation of cellular proliferation by hepatitis B virus X protein. Dis Markers. 2001;17:153-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Shin EC, Shin JS, Park JH, Kim H, Kim SJ. Expression of fas ligand in human hepatoma cell lines: role of hepatitis-B virus X (HBX) in induction of Fas ligand. Int J Cancer. 1999;82:587-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 16. | Wang XZ, Chen XC, Chen YX, Zhang LJ, Li D, Chen FL, Chen ZX, Chen HY, Tao QM. Overexpression of HBxAg in hepatocellular carcinoma and its relationship with Fas/FasL system. World J Gastroenterol. 2003;9:2671-2675. [PubMed] |
| 17. | Terradillos O, de La Coste A, Pollicino T, Neuveut C, Sitterlin D, Lecoeur H, Gougeon ML, Kahn A, Buendia MA. The hepatitis B virus X protein abrogates Bcl-2-mediated protection against Fas apoptosis in the liver. Oncogene. 2002;21:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Chen GG, Lai PB, Chan PK, Chak EC, Yip JH, Ho RL, Leung BC, Lau WY. Decreased expression of Bid in human hepatocellular carcinoma is related to hepatitis B virus X protein. Eur J Cancer. 2001;37:1695-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Gottlob K, Fulco M, Levrero M, Graessmann A. The hepatitis B virus HBx protein inhibits caspase 3 activity. J Biol Chem. 1998;273:33347-33353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 105] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Sirma H, Giannini C, Poussin K, Paterlini P, Kremsdorf D, Bréchot C. Hepatitis B virus X mutants, present in hepatocellular carcinoma tissue abrogate both the antiproliferative and transactivation effects of HBx. Oncogene. 1999;18:4848-4859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 161] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 21. | Shih WL, Kuo ML, Chuang SE, Cheng AL, Doong SL. Hepatitis B virus X protein inhibits transforming growth factor-beta -induced apoptosis through the activation of phosphatidylinositol 3-kinase pathway. J Biol Chem. 2000;275:25858-25864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 159] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Bergametti F, Prigent S, Luber B, Benoit A, Tiollais P, Sarasin A, Transy C. The proapoptotic effect of hepatitis B virus HBx protein correlates with its transactivation activity in stably transfected cell lines. Oncogene. 1999;18:2860-2871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Terradillos O, Pollicino T, Lecoeur H, Tripodi M, Gougeon ML, Tiollais P, Buendia MA. p53-independent apoptotic effects of the hepatitis B virus HBx protein in vivo and in vitro. Oncogene. 1998;17:2115-2123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 136] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Kim H, Lee H, Yun Y. X-gene product of hepatitis B virus induces apoptosis in liver cells. J Biol Chem. 1998;273:381-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 164] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 25. | Su F, Schneider RJ. Hepatitis B virus HBx protein sensitizes cells to apoptotic killing by tumor necrosis factor α. Proc Natl Acad Sci U S A. 1997;94:8744-8749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 251] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Chirillo P, Pagano S, Natoli G, Puri PL, Burgio VL, Balsano C, Levrero M. The hepatitis B virus X gene induces p53-mediated programmed cell death. Proc Natl Acad Sci U S A. 1997;94:8162-8167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 157] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Koike K, Moriya K, Yotsuyanagi H, Shintani Y, Fujie H, Tsutsumi T, Kimura S. Compensatory apoptosis in preneoplastic liver of a transgenic mouse model for viral hepatocarcinogenesis. Cancer Lett. 1998;134:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Schuster R, Gerlich WH, Schaefer S. Induction of apoptosis by the transactivating domains of the hepatitis B virus X gene leads to suppression of oncogenic transformation of primary rat embryo fibroblasts. Oncogene. 2000;19:1173-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Kim YC, Song KS, Yoon G, Nam MJ, Ryu WS. Activated ras oncogene collaborates with HBx gene of hepatitis B virus to transform cells by suppressing HBx-mediated apoptosis. Oncogene. 2001;20:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Tu H, Bonura C, Giannini C, Mouly H, Soussan P, Kew M, Paterlini-Bréchot P, Bréchot C, Kremsdorf D. Biological impact of natural COOH-terminal deletions of hepatitis B virus X protein in hepatocellular carcinoma tissues. Cancer Res. 2001;61:7803-7810. [PubMed] |
| 31. | Takada S, Shirakata Y, Kaneniwa N, Koike K. Association of hepatitis B virus X protein with mitochondria causes mitochondrial aggregation at the nuclear periphery, leading to cell death. Oncogene. 1999;18:6965-6973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 149] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Pollicino T, Terradillos O, Lecoeur H, Gougeon ML, Buendia MA. Pro-apoptotic effect of the hepatitis B virus X gene. Biomed Pharmacother. 1998;52:363-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Bergametti F, Prigent S, Luber B, Benoit A, Tiollais P, Sarasin A, Transy C. The proapoptotic effect of hepatitis B virus HBx protein correlates with its transactivation activity in stably transfected cell lines. Oncogene. 1999;18:2860-2871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Su Q, Schröder CH, Otto G, Bannasch P. Overexpression of p53 protein is not directly related to hepatitis B x protein expression and is associated with neoplastic progression in hepatocellular carcinomas rather than hepatic preneoplasia. Mutat Res. 2000;462:365-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Shirakata Y, Koike K. Hepatitis B virus X protein induces cell death by causing loss of mitochondrial membrane potential. J Biol Chem. 2003;278:22071-22078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |