Published online Apr 1, 2004. doi: 10.3748/wjg.v10.i7.934
Revised: September 17, 2003
Accepted: October 22, 2003
Published online: April 1, 2004
AIM: To investigate the immune function of dendritic cells from both peripheral blood and operated tissues of esophageal carcinoma patients in order to find the relationship between the immune function of dendritic cells and the pathogenesis of esophageal carcinoma.
METHODS: The expression of CD83, CD80, and CD86 on the surface of dendritic cells cultured from the peripheral blood of patients was detected compared with that from health donors using flow cytometry. The ability of dendritic cells to induce T lymphocyte proliferation was evaluated by a liquid scintillation counter. The expression of CD80, CD86, CD83, and S-100 proteins was assessed in esophageal carcinoma tissues using immunohistochemical method.
RESULTS: Compared with those from healthy donors, dendirtic cells cultured from the peripheral blood of patients expressed lower CD80 and CD86. Furthermore, the ability of dendritic cells in patients to induce T lymphocyte proliferation was significantly lower than that of the control group. Compared with the control group, the positive expression ratio and frequencies of CD80, CD86, and S-100 in esophageal carcinoma tissues were significantly down regulated. The expression of CD83 was up-regulated in the pericancerous tissues, but no expression was found in the cancerous nodules.
CONCLUSION: The impaired immune function and the decreased number of dendritic cells cause pathogenesis and progression of esophageal carcinoma.
- Citation: Chen SR, Luo YP, Zhang JK, Yang W, Zhen ZC, Chen LX, Zhang W. Study on immune function of dendritic cells in patients with esophageal carcinoma. World J Gastroenterol 2004; 10(7): 934-939
- URL: https://www.wjgnet.com/1007-9327/full/v10/i7/934.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i7.934
Esophageal carcinoma (EC) is one of the top ten frequently occurred malignant cancers, particularly common in China. Our palace - Chaoshan area, located in the Eastern Guangdong, China, is one of the areas of high-incidence EC with morbidity of 197.82/105 and 81.32/105 world-standardized populations for males and females, respectively[1,2]. Squamous cell carcinoma has been the more common cell-type of EC, accounting for almost 90%. Now the therapy including surgery, chemotherapy, radiation, or a combination for EC is only to palliate the symptoms. However, a 5-year survival rate in patients with esophageal carcinoma is less than 10%[3]. Novel treatment options of EC are urgently needed. Indeed, tumor immunotherapy has evolved specifically to offer several attractive potential advantages. It is strictly autologous with very few side effects. Additionally, once the immune response has started, its efficacy is independent on the localization, type, and proliferative state of tumor cells[4,5].
The aim of tumor immunotherapy is to activate our own immune system to fight the existing tumor. The majority of experimental systems clearly demonstrate that tumor cells are largely defended by CD4+, CD8+ T lymphocytes, or NK cells[6-8]. Lymphocyte T and possibly NK cells, however, require to be activated by antigen-presenting cells (APCs). The dendritic cells (DCs) are one of the most potent APCs in vivo and play crucial roles in the enhancement or regulation of cell-mediated immune reactions[9-11]. Since DCs strongly express various costimulatory and/or adhesion molecules, they can activate even naive T cells in a primary response. The DCs-based approach has been used to establish treatments for several malignant diseases, including B cell lymphoma and melanoma[12-14]. However, the immune functions of DCs are occasionally suppressed under some tumor-bearing states. Thus, the functions of DCs must be assessed in relation to the disease status to further apply DCs as an immunotherapeutic tool. In this study, we detected the expression of CD83, CD80, and CD86 molecules on the surface of DCs cultured from the peripheral blood of patients and healthy donors, and the ability to induce T lymphocyte proliferation. Furthermore immunohistochemical method was used to assess the expression of CD80, CD86, CD83, and S-100 proteins in esophageal carcinoma tissues to find the relationships between the immune function of DCs and the pathogenesis and progression of esophageal carcinoma.
Ten Patients with EC were enrolled in this study. All patients were diagnosed by clinical criteria and confirmed by appropriate histological findings (hematoxylin and eosin staining). To assess the DC function of EC patients, 10 age-matched healthy individuals were assigned as controls.
Esophageal tissue specimens Esophageal tissue specimens were obtained during operation. All patients attended the Second Affiliated Hospital of Shantou University Medical College (Shantou, China) between 2001 and 2002. All samples were fixed in 40 g/L buffered formaldehate, embedded in paraffin, and cut into 5 μm section. Thirty patients with esophageal carcinoma (aged 58-76 yr) were diagnosed by clinical criteria and confirmed by appropriate histological findings (hematoxylin and eosin staining). We took specimens from the esophageal carcinoma tissue (ECT), pericancerous tissue (PCT), and tissue far away from the esophageal carcinoma tissues about 8 cm as the normal esophageal tissue (NET). At the time of surgery, all patients were free from any other tumor therapy.
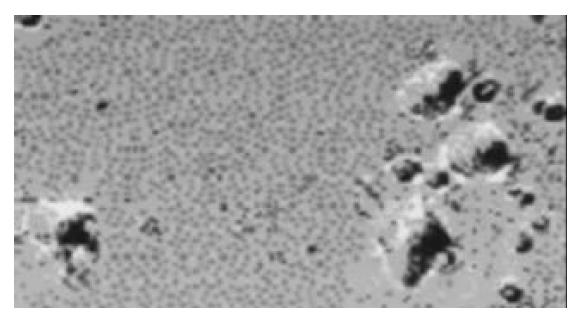
Generation of DCs from peripheral blood mononuclear cells The DCs were generated from peripheral blood mononuclear cells (PBMNC) according to the methods described by Zhu et al[15-19]. The PBMNC were collected from venous blood by Ficoll-Hypaque density-gradient centrifugation. After the PBMNC were suspended in DCs culture medium (RPMI 1640 supplemented with 100 mL/L fetal calf serum, 50 U/mL penicillin, 2 mmol/L L-glutamine, and 50 µmol/L 2-mercaptoethanol), they were placed at 10 mL polystyrene culture plates and stored at 37 °C for 2 h. After incubation, nonadherent cells were removed by gently pipetting with warm RPMI 1640. Adherent cells were supplied with DCs medium containing 800 U/mL of recombinant human GM-CSF and 500 U/mL of recombinant human IL-4, and cultured for 7 d at 37 °C under 50 mL/L CO2. Cells were referred fresh medium containing 800 U/mL of GM-CSF and 500 U/mL of IL-4 every 2 d. After 7 d, the cultures developed an adherent monolayer and clusters of DCs colonies (Figure 1). The DCs yield was defined as the percentage of the obtained DCs numbers to the PBMNC numbers used as the source.
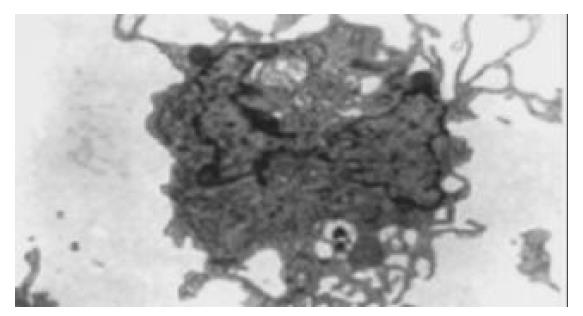
The nonadherent cells were aspirated, washed in PBS, pelleted, and fixed for 30 min in 30 g/L glutaraldehyde in 0.1 mol/L phosphate buffer (pH 7.35). After washing, the cells were postfixed for 1 h in 10 g/L buffered osmium teroxide, washed once in phosphate buffer and 3 times in water, and stained with 5 mL/L aqueous uranyl acetate for 1 h. After dehydration through a series of ethanol dilutions, the cells were pelleted, and embedded in 1:1 Poly/Bed. Thin sections were cut with Diatome diamond knife, mounted 200 mesh nickel guides, and poststained with 50 mL/L methanolic uranyl acetate. Samples were viewed on a Philips 400 transmission electron microscope (Figure 2).
To evaluate the allostimulatroy capacity of DCs, mixed lymphocyte reaction (MLR) was performed. To compare the function between DCs from EC patients (EC-DCs) and those from donors (N-DCs), allogeneic lymphocytes were obtained from the same healthy volunteer. The PBMNC were suspended in DC culture medium and incubated at 37 °C under 50 mL/L CO2 for 1 h, non-adherent cells were gathered as T cells (TCs). After DCs were treated with 50 µg/mL of mitomycin C for 45 min at 37 °C, they were suspended in DC medium and placed at 1 × 102-2 × 104/well on 96-well flat-bottom culture plates. The TCs were mixed with DCs at 2 × 105/well and cultured for 96 h at 37 °C, 50 mL/L CO2. During the last 16 h of incubation, pulse labeling was done with 2.0 µCi/well of [3H] thymidine (MSI, Beijing, China). Assays were performed in triplicate. On day 4, the cells were harvested, and the amounts of [3H] thymidine incorporated to responder cells were counted with a beta counter. The ratio of MLR was determined by the ratios of cpm between EC-DC and N-DC in the presence of the same reagents at a TCs/DCs ratio of 10 to 1.
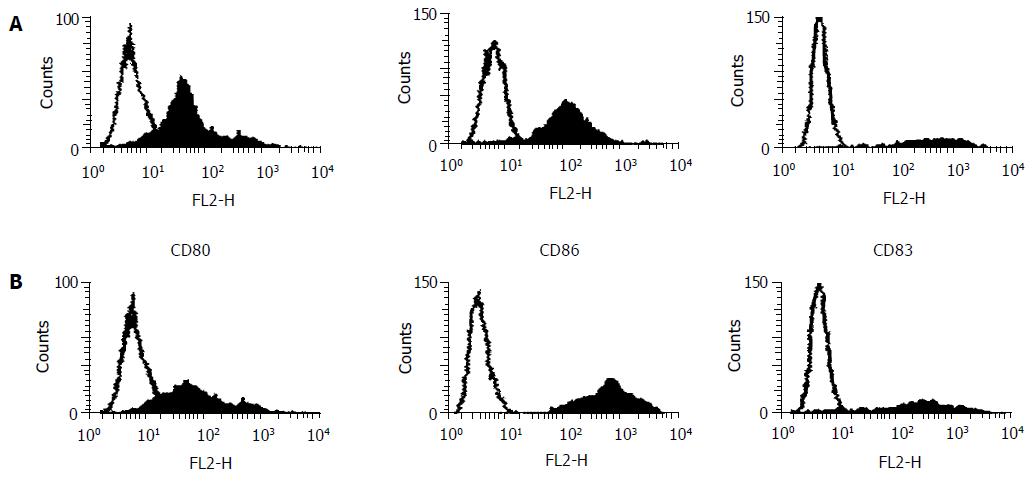
After 7 d of culture, DCs were harvested, and their surface molecule expression was analyzed using FACS (Becton Dickinson Immunocytometry Systems, San Jose, CA). In each step of the staining, 5 × 104 cells were stored with specific antibodies (Abs) for 30 min at 4 °C in 50 µL of PBS containing 20 mL/L of bovine serum albumin and 1 mL/L of sodium azide. For the staining of mouse monoclonal anti-human CD80, CD83, or CD86 (PharMingen, San Diego, CA), fluorescein isothiocyanate (FITC)-conjugated goat monoclonal anti-mouse IgG was used according to the procedure of indirect immunofluorescence staining. Isotypic Ab (purified mouse IgG) was substituted for specific Abs to obtain a negative control. After staining, all cells were fixed with 10 mL/L paraformaldehyde (Sigma), and analyzed with the FACStarP1 (Becton Dickinson) using a single argon laser. For the comparison of CD83 and costimulators, the mean fluorescence intensity of stained DCs (MFIs) and that of controls (MFIc) was measured using a Consort 30 software program (Becton Dickinson). The degree of surface molecule expression was estimated as the ratio of MFIs to MFIc (MFIs/MFIc) and expressed as the net fluorescence intensity (NFI). All samples were assayed in duplicate.
Immunohistochemical staining of CD80, CD86, and S100 proteins Immunohistochemical staining was performed. Rabbit anti-human monoclonal antibodies to CD80, CD86 and S100 proteins (PharMingen International) were used as primary antibodies (Ab1). The antigen-Ab1 complex was then incubated with biotinylated secondary antibody followed by horseradish peroxidase-avidin. Staining procedure was done according to the introduction of the agent box, SP method. A known sample from a patient with breast cancer was used as a positive control, and the staining for negative controls was followed the method described above, except for incubation with PBS instead of the primary antibody. Colorization was performed by diaminobenzidine (DAB)-hydrogen peroxide as a chromogen.
Immunohistochemical staining of CD83 The staining of CD83 was performed with some modifications, according to a previously described method[19]. Formalin-fixed, paraffin-embedded specimens were washed three times in PBS and treated with pepsin (5 mg/mL in 0.01 mol/L HCl, Zhongshan, Beijing, China) for 20 min at 37 °C before staining for CD83. The specimens were then treated with normal goat serum for 20 min to block non-specific binding. The monoclonal antibody with appropriate dilution was then added. Tissue sections were treated with 0.3 mL/L methanol-hydrogen peroxide to inactivate endogenous peroxidase. The sections then were reincubated with biotinylated goat anti-mouse immunoglobulin at room temperature for 1 h. After a wash in PBS, sections were soaked in alkaline phosphatase-conjugated streptavidin, washed, and New Fuchsin was used as a chromogen. A known sample from patients with primary biliary cirrhosis was used as a positive control. The staining of negative controls was performed using PBS instead of primary antibody.
Enumeration of positive staining cells The numbers of CD80-, CD86-, S100-, and CD83-positive cells in the whole specimen were determined. The prevalence of CD80, CD86, and S100 protein-positive cells were shown as the ratio of a total 100 infiltrating cells. The prevalence of CD83 positive cells was shown as total numbers of cells/specimen.
The data were expressed as mean ± SD. The statistical analyses were done by unpaired and paired t-tests, when indicated. Mann-Whitney’s U-test and the Wilcoxon rank-sum test were also used when unpaired and paired t-tests were not indicated, respectively. P-values less than 0.05 were considered to indicate statistical significance. Statistical calculations were performed using the SPSS 10.0 statistical program.
After 7 d of culture under GM-CSF and IL-4, the cells from patients or volunteers exhibited DC morphology with veiled process and dendrites. Through the observation from transmission electron microscopy, the cell surface was irregular but lacked the discrete microvilli and ruffles (Figures 1, 2). The cells were free polysomes and ribosomes, and lysosomes were scanty. These results showed that the generated cells from both patients and donors were morphological and compatible with DCs. The expression of CD80 and CD86 in EC patients was weaker than that in volunteers, but no the expression of CD83 was not different between EC patients and volunteers (Table 1 and Figure 3). The yield of DCs was not different between patients and donors (5 ± 3% vs 5 ± 1%).
In MLRs, the degree of T cell proliferation from EC-DCs was significantly lower than that from N-DCs. The reduced allogeneic response from EC-DCs was confirmed with the comparison of the cpm at a TCs/DCs ratio of 10 to 1 with large numbers of subjects (P < 0.01) (Table 1). Since the responders were identical in each series of the comparisons, the difference in TCs proliferation mainly depended on the DCs difference.
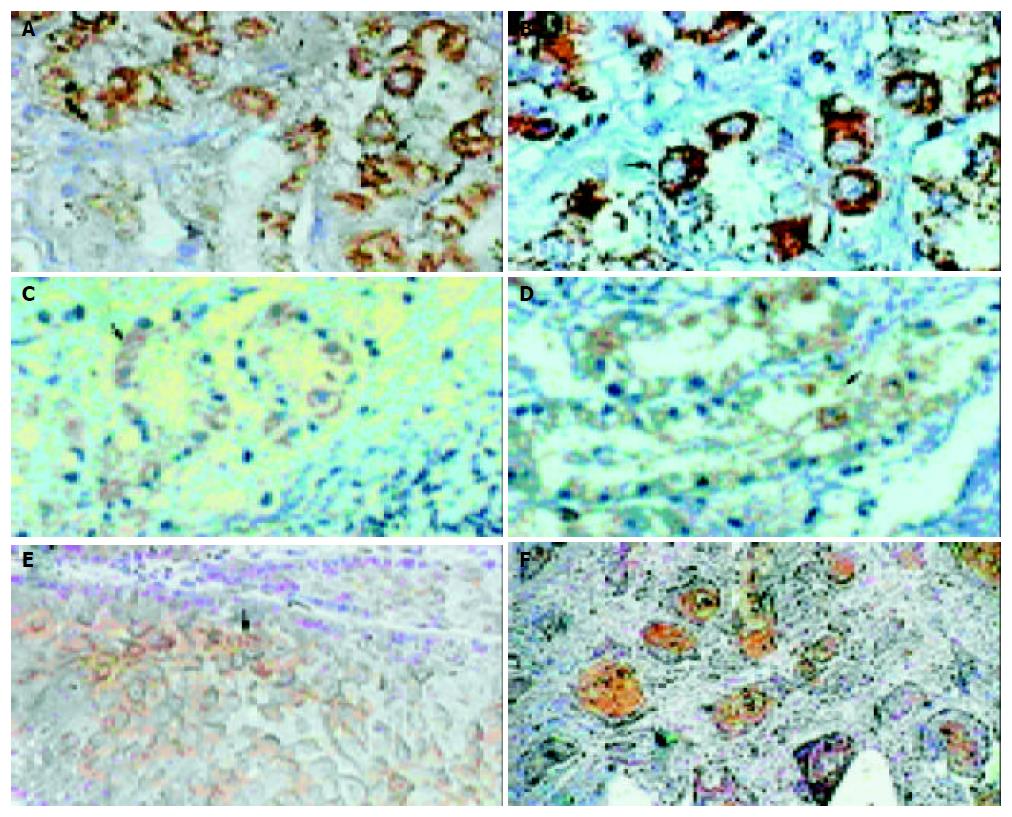
The expression of CD80 and CD86 could be detected in the nucleus and cytoplasm but not on the cell membrane for all the specimens. The expression of CD80 and CD86 in the nucleus was found randomly. The expression in the cytoplasm was located in the necrotic area and around with infiltrated lymphocytes (Figure 4A, B, C, D). The prevalence of positive expression of CD80 and CD86 was lower in ECT than that in NET (P < 0.01), but there was no significant difference between PCT and NET (Table 2).
The S100 protein-positive cells were diffusely detected in infiltrating cells of esophageal mucous membrane and epithelium tissues. In ECT, they were localized in cancer cells and cancer nodules; only little could be detected in the tissues away from cancer tissues (Figure 4E, F). Contrast to NET, ECT expressed lower S100-positive cells (P < 0.05) (Table 2).
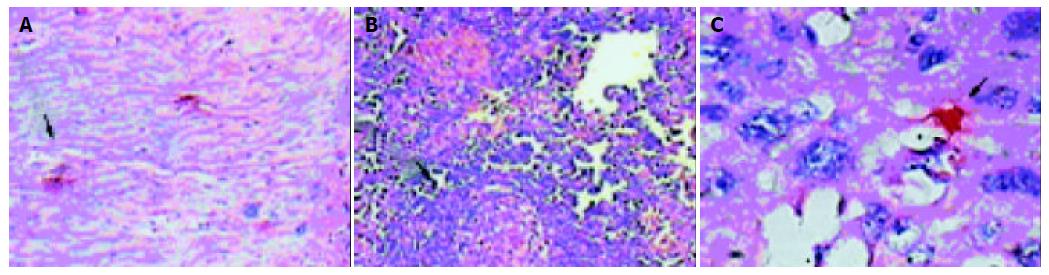
The CD83-positve cells were detected in infiltrating cells in PCT. The NET did not show any positive staining for CD83. In ECT, CD83-positive cells could only be dispersedly detected surround cancer nodules, but there were no CD83-positive activated DCs in cancer nodules (Figure 5A, B, C). Contrast to PCT, ECT expressed lower CD83-positive cells (P < 0.05) (Table 3).
To activate naive T cells, DCs can uptake, process, and present antigens via their MHC molecules. In addition, T cell activation requires engagement of costimulatory receptors on the T cells. The DCs are at the center of the developing tumor-specific immune response, and are involved both in the initiation of tumor-specific immunity and the generation of immune effector functions. Many observations have suggested that DCs in tumors are functionally impaired[19-24].
In this study, we compared the functions of DCs generated from EC patients with those from healthy volunteers. Our results showed that the allogeneic MLR from EC-DCs was lower than that from N-DCs. With regard to the APC dysfunction in EC-DCs, the decreased expression of costimulators might be involved. Among the examined costimulators, lower expression of CD80 and CD86 was found in EC-DCs. The importance of the CD80/CD86-CD28 system in T cell responses has been well demonstrated[10,20,21,29].
The CD80 and CD86, members of the immunoglobulin super gene family, are encoded by separate genes and provide costimulatory function for APC-dependent T-cell activation both in vivo and in vitro[25-28]. A previous study reported that most tumor tissues, like those from the nonhematopoietic origin, did not express costimulatory molecules, which would render T cells unresponsive for the specific antigens[20]. Recent studies demonstrated that B7 expression of tumor cells resulted in increased tumor immunogenicity, enhanced generation of allogeneic and antilogous tumor reactive CTL. The decrease, abrogation or loss of CD80 and CD86 were detected both from EC-DCs and specimens from EC patients in this study, which might have profound implications for the down-regulation of the immune system and for enhancing the progression of esophageal carcinoma.
Furthermore, both the expression of CD80 and CD86 in EC sections could only be detected in the nucleus and cytoplasm but not on the cell membrane, suggesting the impaired transmembrane transfusion of CD80 and CD86 molecules. This interesting phenomena was similar to the B7 molecule expression in renal carcinoma and hepatocarcinoma[29,30].
The lower frequency of S100-positive cells in EC indicated the less DCs during esophageal cacinogenesis. However, the S100 protein family contains 21 members, and so far the physiological implication of its function is confusing[19]. Therefore, the significance of positive S100 protein is not clearly known.
Because CD83-positive DCs could not be detected in most of the normal esophagus, their existence in the tissues from EC patients indicated the maturation and activation of immature DCs. Alternatively, it was also possible that the activated CD83-positive DCs infiltrated into the esophagus in EC patients through the circulation in spite of having almost similar frequencies of CD83-positive DCs in peripheral blood form both healthy donors and EC patients. Most strikingly, all CD83-positive DCs were localized in the pericancerous tissues, and there were no CD83-positive DCs in any cancer nodules.
What is the significance of absence of activated DCs in cancer nodules form patients with EC? Activated DCs present the antigenic epitope to T cells and induce its activation, which are the most strong inducers for IL-12 and specific cytotoxic T lymphocytes during carcinogenesis[31,32]. In the absence of activated DCs in cancer nodules, normal immune surveillance against EC may be hampered due to defective production of tumor -specific lymphocytes. Again, tumor-specific lymphocytes would not be able to function and survive in the absence of activated DCs.
In summary, the impaired immune functions of DCs in patients with EC, including lower amount of DCs, decreased expression of CD80/86, minimally activated DCs, and complete absence of activated DCs in cancer nodules, are correlated to the pathogenesis and progression of EC.
Edited by Chao JCJ and Xu FM
| 1. | Simchuk EJ, Alderson D. Oesophageal surgery. World J Gastroenterol. 2001;7:760-765. [PubMed] |
| 2. | Dan HL, Bai Y, Meng H, Song CL, Zhang J, Zhang Y, Wan LC, Zhang YL, Zhang ZS, Zhou DY. A new three-layer-funnel-shaped esophagogastric anastomosis for surgical treatment of esophageal carcinoma. World J Gastroenterol. 2003;9:22-25. [PubMed] |
| 3. | Zhou J, Zhao LQ, Xiong MM, Wang XQ, Yang GR, Qiu ZL, Wu M, Liu ZH. Gene expression profiles at different stages of human esophageal squamous cell carcinoma. World J Gastroenterol. 2003;9:9-15. [PubMed] |
| 4. | Liu XJ, Wang BM. Biotherpy of esophageal carcinoma. Shijie Huaren Xiaohua Zazhi. 2000;8:1027-1029. |
| 5. | Chen KN, Xu GW. Diagnosis and therapy of esophageal carcinoma. Shijie Huaren Xiaohua Zazhi. 2000;8:196-202. |
| 6. | Zhang J, Zhang JK, Zhuo SH, Chen HB. Effect of a cancer vaccine prepared by fusions of hepatocarcinoma cells with dendritic cells. World J Gastroenterol. 2001;7:690-694. [PubMed] |
| 7. | Wykes M, MacPherson G. Dendritic cell-B-cell interaction: dendritic cells provide B cells with CD40-independent proliferation signals and CD40-dependent survival signals. Immunology. 2000;100:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Tascon RE, Soares CS, Ragno S, Stavropoulos E, Hirst EM, Colston MJ. Mycobacterium tuberculosis-activated dendritic cells induce protective immunity in mice. Immunology. 2000;99:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Aiba S, Manome H, Yoshino Y, Tagami H. In vitro treatment of human transforming growth factor-beta1-treated monocyte-derived dendritic cells with haptens can induce the phenotypic and functional changes similar to epidermal Langerhans cells in the initiation phase of allergic contact sensitivity reaction. Immunology. 2000;101:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Mogi S, Sakurai J, Kohsaka T, Enomoto S, Yagita H, Okumura K, Azuma M. Tumour rejection by gene transfer of 4-1BB ligand into a CD80(+) murine squamous cell carcinoma and the requirements of co-stimulatory molecules on tumour and host cells. Immunology. 2000;101:541-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Li MS, Yuan AL, Zhang WD, Chen XQ, Tian XH, Piao YJ. Immune response induced by dendritic cells induce apoptosis and inhibit proliferation of tumor cells. Shijie Huaren Xiaohua Zazhi. 2000;8:56-58. |
| 12. | Sun J, Zhang J, Chen J, Chen H, Chew Y, Chen J. In vitro study on the morphology of human blood dendritic cells and LPAK cells inducing apoptosis of the hepatoma cell line. Chin Med J (Engl). 2001;114:600-605. [PubMed] |
| 13. | Li MS, Yuan AL. Study about immunology function of den-dritic cells from liver patients. Chin Oncology. 1998;8:85-87. |
| 14. | Yu JW, Wang GQ, Lu SL. Study of immune function of periph-eral blood dendritic cells from chronic hepatitis B patients. Chin Infer Dis. 2001;19:144-147. |
| 15. | Zhu XJ, Cao XT, Yu YZ, Chen GY, Wan T, Ma SH, Tang H, Zhang WP. Generation of dendritic cells from human periph-eral blood. Chin J Cancer Biother. 1997;4:302-306. |
| 16. | Wang YD, Xie W, Qiu YH, Gu ZJ, Zhang XG. The study of dendritic cells induced by cytokines from different adherent cells in vitro. Shanghai Immunol J. 2000;20:140-144. |
| 17. | Li YQ, Zeng BH. In vitro culture and anti-cancer immunity of dendritic cells. Chin J Cancer Prev Treat. 2001;8:78-80. |
| 18. | Lutz MB, Kukutsch N, Ogilvie AL, Rössner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2380] [Cited by in RCA: 2515] [Article Influence: 96.7] [Reference Citation Analysis (0)] |
| 19. | Chen S, Akbar SM, Tanimoto K, Ninomiya T, Iuchi H, Michitaka K, Horiike N, Onji M. Absence of CD83-positive mature and activated dendritic cells at cancer nodules from patients with hepatocellular carcinoma: relevance to hepatocarcinogenesis. Cancer Lett. 2000;148:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Woods GM, Doherty KV, Malley RC, Rist MJ, Muller HK. Carcinogen-modified dendritic cells induce immunosuppression by incomplete T-cell activation resulting from impaired antigen uptake and reduced CD86 expression. Immunology. 2000;99:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Avigan D. Dendritic cells: development, function and potential use for cancer immunotherapy. Blood Rev. 1999;13:51-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 71] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Zhang JK, Sun JL, Chen HB, Zeng Y, Qu YJ. Influence of granulocyte macrophage colony stimulating factor and tumor necrosis factor on anti-hepatoma activities of human dendritic cells. World J Gastroenterol. 2000;6:718-720. [PubMed] |
| 23. | Zhang JK, Li J, Chen HB, Sun JL, Qu YJ, Lu JJ. Antitumor activities of human dendritic cells derived from peripheral and cord blood. World J Gastroenterol. 2002;8:87-90. [PubMed] |
| 24. | Li MS, Yuan AL, Zhang WD, Chen XQ, Zhang YL, Zhou DY. Study on immune function of dendritic cells in patients with colorectal neoplasms. Shijie Huaren Xiaohua Zazhi. 1999;7:429. |
| 25. | Zhang J, Xu J, Jia S. [Infiltration of dendritic cells into cervical lymph nodes in laryngeal carcinoma]. Zhonghua Erbiyanhouke Zazhi. 2000;35:472-474. [PubMed] |
| 26. | Zhang XZ, An HL, Zhang XB. Immunohistochemical study of S-100 protein-positive dendritic cells in esophageal carcinoma. J Xi'an Med Univ. 1998;19:215-218. |
| 27. | Ito T, Amakawa R, Inaba M, Ikehara S, Inaba K, Fukuhara S. Differential regulation of human blood dendritic cell subsets by IFNs. J Immunol. 2001;166:2961-2969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 236] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 28. | Xing LH, Wang FS, Liu MX, Zhu CL. Dendritic cells and liver diseases. Shijie Huaren Xiaohua Zazhi. 2000;8:1276-1279. |
| 29. | Zai SH, Liu JB, Zhu P, Wang YH. The expression of CD80 and CD86 in hepatocarcinoma and cirrhosis tissues. J Fourth Mil Med Univ. 2001;21:26-27. |
| 30. | Wang HL, Xiu AP, Tan SF, Wu J, Xie H, Xiao J. The expression of CD80 and CD86 in renal carcinoma. Anat Res. 2001;23:88-90. |
| 31. | Fan P, Wu ZY, Wang S. An investigation on the phenotype of cultured dendritic cells from the peripheral blood of patients with breast cancer. J NanJing Med Univ. 2002;16:115-118. |
| 32. | Hao MW, Liang YR, Liu YF, Liu L, Wu MY, Yang HX. Transcription factor EGR-1 inhibits growth of hepatocellular carcinoma and esophageal carcinoma cell lines. World J Gastroenterol. 2002;8:203-207. [PubMed] |