INTRODUCTION
Gram-negative bacteria caused sepsis remains an important cause of morbidity and mortality in septic and endotoxemic patients. Lipopolysaccharide (LPS), or endotoxin, a major component of the outer surface of Gram-negative bacteria, is a potent activator of cells of the immune and inflammatory systems, including macrophages, monocytes and endothelial cells[1], and contributes to the systemic changes seen in septic shock[1-4]. The endotoxic shock syndrome is characterized by systemic inflammation, multiple organ damage, circulatory collapse and death[1,2].
The important role of the intestinal mucosa in the inflammatory and metabolic responses to sepsis, severe injury and other critical illnesses has been increasingly recognized during the last decade. Thus, there is evidence that the gut mucosa becomes the site for production of various inflammatory cytokines[5,6] and other yet unidentified substances that may influence not only the mucosa itself but also the function and integrity of remote organs and tissues[7,8]. Indeed, the gut mucosa has been proposed to be the “motor” of multiple organ failure in critical illness[9]. Besides, sepsis and severe injury are also associated with loss of mucosal integrity, resulting in increased permeability and bacterial translocation. These changes may accelerate the development of multiple organ failure[10].
Ketamine, an intravenous anesthetic, has been advocated for anesthesia in septic or severely ill patients because of its cardiovascular stimulating effects[11,12]. And several previous studies reported that ketamine could suppress LPS-induced tumor necrosis factor alpha (TNF-α) production in the serum and reduced mortality in carrageenan-sensitized endotoxin shock mice[13,14]. However, few studies were undertaken to investigate the protective effect of ketamine on the inflammatory response in the intestine during septic shock in vivo. Since local produced cytokines were regarded as the contributing factors in tissue damage during sepsis[15-17]. And nuclear factor kappa B (NF-kappa B) was verified to be an inducible transcription factor that was required for the transcription of some proinflammatory cytokines such as TNF-α, interleukin 6 and interleukin 8 (IL-6 and IL-8)[18]. Our previous study indicated that ketamine could inhibit endotoxin induced NF-kappa B and TNF-αin vitro[19]. Therefore, this study was to investigate whether ketamine could suppress endotoxin-induced NF-kappa B activation and proinflammatory cytokines in the intestine in vivo in order to define a possible mechanism of the anti-inflammatory effect of ketamine.
MATERIALS AND METHODS
Animals and treatment
Adult male Wistar rats (250-300 g body mass) used in this experiment were purchased from Shanghai Animal Center, Shanghai, China. The rats were exposed each day to 12 h of light and darkness respectively. The experimental protocol followed the institution’s criteria for the care and use of laboratory animals in research. Further, all animals received humane care in compliance with Institutional Animal Care Committee.
Experimental protocol
The Wistar rat endotoxemia model was established by injection with a dose of LPS (5 mg/kg, Escherichia coli O111: B4) (Sigma Chemical Co., USA) via the tail vein. Then all animals were immediately treated with different doses of ketamine (0.5, 5, 50 mg/kg) or normal saline (10 mL/kg) intraperitoneally (ip). Endotoxin and ketamine were diluted with normal saline at different concentrations so as to inject them into the rats at the same volume (10 mL/kg ). After 1, 4 or 6 h, animals were killed, and tissues from the intestine was removed and kept in liquid nitrogen for later use. We used six rats in every time point of each group.
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts of the intestine tissue was prepared by hypotonic lysis followed by high salt extraction[20-22]. EMSA was performed using a commercial kit (Gel Shift Assay System; Promega, Madison, WI) as previously described. The NF-kappa B oligonucleotide probe, (5’-AGTTGAGGGGACT TTCCCAGGC-3’), was end-labeled with [γ-32P] ATP (Free Biotech, Beijing, China) with T4-polynucleotide kinase. Nuclear protein (80 μg) was preincubated in 9 μL of a binding buffer, consisting of 10 mmol/L Tris-Cl, pH 7.5, 1 mmol/L MgCl2, 50 mmol/L NaCl, 0.5 mmol/L EDTA, 0.5 mmol/L DTT, 40 mL/L glycerol, and 0.05 g/L of poly-(deoxyinosinic deoxycytidylic acid) for 15 min at room temperature. After addition of the 1 μL 32P-labeled oligonuleotide probe, the incubation was continued for 30 min at room temperature. Reaction was stopped by adding 1 μL of gel loading buffer, and the mixture was subjected to non-denaturing 40 g/L polyacrylamide gel electrophoresis in 0.5 × TBE buffer. The gel was vacuum-dried and exposed to X-ray film (Fuji Hyperfilm) at -70 °C.
Reverse-transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted with TriPure Isolation Reagent (Roche Molecular Biochemicals, Switzerland) and quantified by absorption at 260 nm. Reverse-transcription (RT) was implemented using Reverse Transcription System (Promega, WI, USA) according to the protocol. We used glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as normalization control. The sequences of the primers were: TNF-α (sense) CACCACGCTCTTCTGTCTACTGAAC, (antisense) CCGGACTCCGTGATGTCTAAGTACT; IL-6 (sense) GACTGATGTTGTTGACAGCCACTGC, (antisense) TAGCC ACTCCTTCTGTGACTCTAACT; GAPDH (sense) C A C G G C A A G T T C A A T G G C A C A , ( antisense ) GAATTGTGAGGGAGAGTGCTC. A total volume of 100 μL reaction contained 2 μL of RT product, 1.5 mmol/L MgCl2, 2.5 U Taq DNA polymerase, 100 μmol/LdNTP, 0.1 μmol/L primer and 1 × Taq DNA polymerase magnesium-free buffer (Promega, WI, USA). Then the reaction mixture was overlaid with two drops of mineral oil (Sigma Chemical Co., USA) and incubated in thermocycler (MiniCycler PTC 150, MJ Research Inc, USA) programmed to pre-denature at 95 °C for 2 min, denatured at 95 °C for 1 min, annealed at 60 °C for 1 min and extended at 72 °C for 2 min for a total of 30 cycles. The last cycle was followed by a final incubation at 72 °C for 5 min and cooled to 4 °C. The polymerase chain reaction products were 546 bp (TNF-α), 509 bp (IL-6) and 970 bp (GAPDH) respectively. Then they were electrophoresed on a 15 g/L agarose gel stained with ethidium bromide. The gel was captured as a digital image and analyzed using Scion Image software (Maryland, USA). Values in each sample were normalized with GAPDH control.
Enzyme-linked immunoadsordent assay (ELISA)
TNF-α and IL-6 in the intestine were measured using commercially available enzyme-linked immunoassay kits (Diaclone USA for TNF-α; Biosource USA for IL-6) according to the test protocol. Values were expressed as pg per milligram protein (pg/mg prot).
Statistics and presentation of data
Data were expressed as mean ± SE. Statistical significance was determined by one-way ANOVA using SPSS 10.0. A value of P < 0.05 was considered significant.
RESULTS
NF-kappa B activation in the intestine
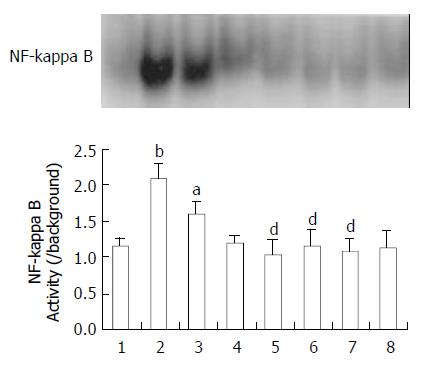
EMSA experiments were performed to examine the effect of ketamine on the activation of NF-kappa B induced by endotoxin. As shown in Figure 1, NF-kappa B activation in the intestine was increased after endotoxin challenge as compared with unstimulated group. The activity of NF-kappa B was in a time dependant manner after endotoxin injection. Ketamine inhibited NF-kappa B activation at three (0.5, 5, and 50 mg/kg) dosing levels (P < 0.05, as compared with endotoxin group) (Figure 1).
Figure 1 Activation of NF-kappa B in the intestine.
Normal saline treatment (Lane 1). 1, 4, 6 h after endotoxin challenge (Lane 2, 3, 4), endotoxin plus ketamine (0.5, 5, 50 mg·kg-1) (Lane 5, 6, 7), ketamine only (50 mg·kg-1) (Lane 8) aP < 0.05 vs Lane 1; bP < 0.01 vs Lane 1; dP < 0.01 vs Lane 2.
TNF-α mRNA expression by endotoxin challenge and the protective effect of ketamine
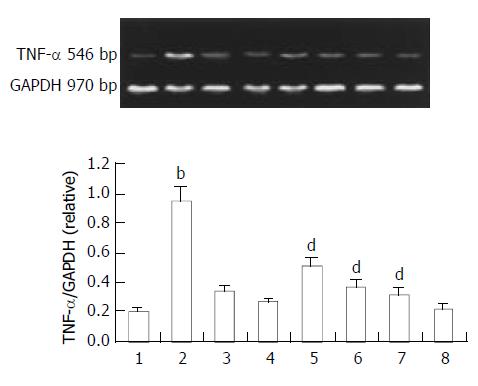
TNF-α sustained a baseline level in normal rats. Endotoxin caused a transient elevation of TNF-αmRNA in the intestine. This activity increased with time reaching a maximum 1 h after sepsis. Ketamine was administered intraperitoneally soon after endotoxin challenge. TNF-α gene expression was analyzed 1 h later since TNF-α could reach the maximum level about 1 h later. Ketamine suppressed TNF-α expression in a dose dependent manner. We found that ketamine at a dose of 0.5 mg/kg could suppress TNF-α expression significantly. This dosage was far below clinical anesthetic level (Figure 2).
Figure 2 Expression of TNF-α in the intestine.
Normal saline treatment (Lane 1). 1, 4, 6 h after endotoxin challenge (Lane 2, 3, 4), endotoxin plus ketamine (0.5, 5, 50 mg·kg-1.) (Lane 5, 6, 7), ketamine only (50 mg·kg-1) (Lane 8); bP < 0.01 vs Lane 1; dP < 0.01 vs Lane 2.
IL-6 expression in intestine by endotoxin challenge and the protective effect of ketamine
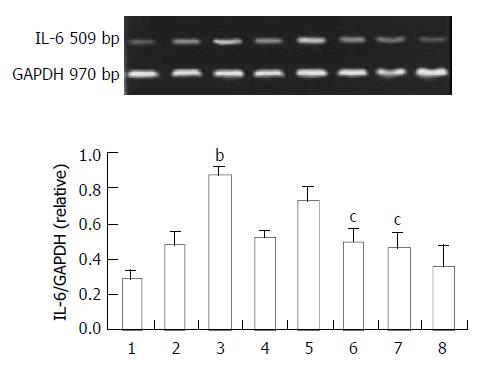
The IL-6 expression of the small intestine is shown in Figure 3. Endotoxin also enhanced IL-6 expression in the intestine. However the peak time was 4 h after endotoxin challenge. We observed the protective effect of ketamine at this peak time. Ketamine suppressed IL-6 expression in a dose dependent manner. Unlike TNF-α, the minimal dosage at which ketamine could suppress IL-6 significantly was 5 mg/kg. This was within clinical anesthetic range (Figure 3).
Figure 3 Expression of IL-6 in the intestine.
Normal saline treatment (Lane 1). 1, 4, 6 h after endotoxin only (Lane 2, 3, 4), endotoxin plus ketamine (0.5, 5, 50 mg/kg) (Lane 5, 6, 7), ketamine only (50 mg/kg) (Lane 8); bP < 0.01 vs Lane 1; cP < 0.05 vs Lane 3.
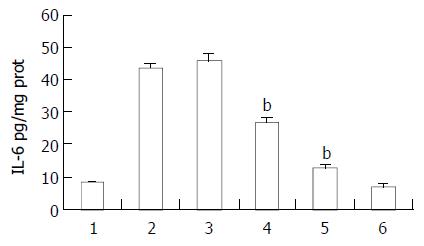
Effect of ketamine on TNF-α and IL-6 production in intestine homogenates after endotoxin stimulation
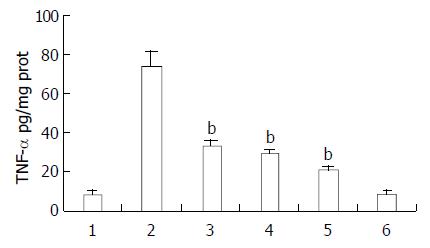
Ketamine suppressed endotoxin-induced TNF-α and IL-6 production in a dose dependent manner. Ketamine beyond the concentration of 0.5 mg/kg could inhibit TNF-α production, however the minimal dosage at which ketamine suppressed IL-6 significantly was 5 mg/kg. This was within clinical anesthetic range (Figures 4 and 5).
Figure 4 Protective effect of ketamine on the TNF-α production in the intestine.
All of the values were obtained 1 h after sepsis. Lane 1 normal saline; Lane 2 endotoxin (5 mg/kg); Lane 3 endotoxin (5 mg/kg) plus ketamine (0.5 mg/kg); Lane 4 endotoxin (5 mg/kg) plus ketamine (5 mg/kg); Lane 5 endot-oxin (5 mg/kg) plus ketamine (50 mg/kg); Lane 6 ketamine only (50 mg/kg) bP < 0.01 vs Lane 2.
Figure 5 Protective effect of ketamine on the IL-6 production in the intestine.
All of the values were obtained 4 h after sepsis. Lane 1 normal saline; Lane 2 endotoxin (5 mg/kg); Lane 3 endotoxin (5 mg/kg) plus ketamine (0.5 mg/kg); Lane 4 en-dotoxin (5 mg/kg) plus ketamine (5 mg/kg); Lane 5 endot-oxin (5 mg/kg) plus ketamine (50 mg/kg); Lane 6 ketamine only (50 mg/kg) bP < 0.01 vs Lane 2.
DISCUSSION
Our laboratory and others have demonstrated that ketamine could suppress endotoxin-induced some proinflammatory cytokines in vitro[23]. However it is to be determined in complex in vivo studies. We assessed the cytokines and transcriptional factor NF-kappa B in the intestine because of the important status of the intestine in sepsis or systemic inflammation reaction syndrome (SIRS). The intestine was not only the passive organs injured by sepsis but participation in the pathogenesis of SIRS[5,6].
TNF-α is regarded as the most important proinflammatory cytokine, which is released early after an inflammatory stimulus[24]. And IL-6, which is elevated after TNF-α, contributes to both morbidity and mortality in conditions of “uncontrolled” inflammation[25]. Among the cytokines produced in the intestinal mucosa during inflammation, TNF-α and IL-6 are particularly important because of its multiple biological effects both in the intestine and in other organs and tissues. In this study, we demonstrated that ketamine suppressed both endotoxin-induced TNF-α and IL-6 expression and production in the intestine. TNF-α was the first cytokine expressed after endotoxin stimulation and later IL-6, which was consistent with several previous reports[24,25]. Studies had demonstrated that ketamine could suppress endotoxin-induced cytokines in vitro. However, proinflammatory cytokines just like TNF-α and IL-6 were not merely stimulated by endotoxin in vivo. Therefore, our experimental protocol was more physiological and closer to clinical condition.
NF-kappa B is associated in the cytoplasm with its inhibitory subunit, inhibitory kappa B (IκB), which prevents NF-kappa B from translocating into the nucleus. Endotoxin can induce the phosphorylation and degradation of IκB. Many effector genes including those encoding cytokines (TNF-α and IL-6) are in turn regulated by NF-kappa B[26]. To determine whether ketamine could inhibit NF-kappa B activation, we did EMSA to detect NF-kappa B activity in the intestine. We found a constitutive activation of NF-kappa B in intestine. Endotoxin could enhance NF-kappa B activation in the intestine and it was most significant 1 h later. Although we had previously demonstrated that ketamine could inhibit NF-kappa B activation in peripheral blood mononuclear cell (PBMC) after endotoxin challenge in vitro. It was to be studied whether ketamine had this effect in vivo. In our experiment, we found ketamine could inhibit NF-kappa B activation. However it was not in a dose dependent manner. We did not found any NF-kappa B activity changes in the group administered ketamine (50 mg/kg ) only, which excluded ketamine itself had any effect on NF-kappa B activity.
As the rats were not anesthetized during the whole experiment, we did not monitor the arterial pressure and pulse rate to confirm septic shock. Because the drug studied in our investigation was ketamine, just an anesthetic drug. To exclude any other anesthetic drug disturbance, we had to give up that monitoring. However, this septic model was successfully used in many other researches[27-29]. In addition, we did find that the rats were dispirited with piloerection and diarrhea, which indicated the septic shock indirectly.
The dosage of ketamine used in this study was from 0.5 to 50 mg/kg, which covered the clinical range. Roytblat et al[30] reported that a single dose of ketamine 0.25 mg/kg administered before cardiopulmonary bypass suppressed the increase in serum IL-6 during and after coronary artery bypass surgery. However, other studies demonstrated such a small dose of ketamine did not suppress IL-6 production[25]. The reason was not clear. In this study, only ketamine reaching a dose of 5 mg/kg could suppress IL-6 production in the intestine. There were perhaps some differences between human being and animals or between in vitro and in vivo studies. We found 0.5 mg/kg ketamine suppressed TNF-α production, which was in accordance with those in vitro studies[25].
In conclusion, we demonstrated that ketamine could suppress endotoxin-induced TNF-α and IL-6 expression and production in the intestine. And this suppressive effect might act through inhibiting NF-kappa B. Further study is required to elucidate the mechanism of ketamine action.