Published online Feb 1, 2004. doi: 10.3748/wjg.v10.i3.400
Revised: March 1, 2003
Accepted: March 5, 2003
Published online: February 1, 2004
AIM: To investigate the in vitro effects of suicide gene therapy system of herpes simplex virus thymidine kinase gene (HSV-TK) in combination with the treatment of nucleotide analog-ganciclovir (GCV) on human pancreatic cancer, and to provide a novel clinical therapeutic method for human pancreatic cancer.
METHODS: We used a replication defective recombinant retrovirus vector GINaTK (bearing HSV-TK gene) to make packaging cell PA317 produce progeny virions. We then transferred the HSV-TK gene to target cells SW1990 using these progeny virions, and treated these gene-modified tumor cells with GCV to study the sensitivity of the cells to GCV and their bystander effects by routine MTT-method.
RESULTS: Packaging cell PA317/TK was successfully constructed, and we acquired SW1990/TK through virus progeny infection. These gene-modified pancreatic cancer cells were sensitive to the treatment of GCV compared with unmodified tumor cells (t = 4.15, n = 10, P < 0.0025). We also observed a remarkable bystander effect by mixing two kinds of cells at different ratio.
CONCLUSION: Our data demonstrate that HSV-TK/GCV suicide gene therapy system is effective for treating experimental human pancreatic cancer, which is largely resistant to the common therapies, so the suicide gene therapy system may be a potential treatment approach for pancreatic cancer.
- Citation: Wang J, Lu XX, Chen DZ, Li SF, Zhang LS. Herpes simplex virus thymidine kinase and ganciclovir suicide gene therapy for human pancreatic cancer. World J Gastroenterol 2004; 10(3): 400-403
- URL: https://www.wjgnet.com/1007-9327/full/v10/i3/400.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i3.400
Pancreatic cancer is an aggressive malignancy with less than 5% of the patients alive at 5 years and 92% of the patients dead at 2 years[1,2]. Despite of the development in the three routine therapeutic methods of surgery[3], chemotherapy, and radiotherapy, the cure rate for pancreatic cancer has improved only minimally, and the overall survival of patients remains dismal. Its prognosis is extremely poor with current modes of treatment[4,5]. Therefore, it is urgent to develop effective approaches to this lethal disease. With great progresses of gene therapy in recent years[6], much of interest and effort have been focused on the treatment of pancreatic cancer. The use of pro-drug-activating genes is a promising approach for cancer gene therapy, especially herpes simplex virus thymidine kinase gene (HSV-TK) in combination with ganciclovir (GCV), which is currently used in gene therapy-based experimental trials for cancer treatment, and in clinical treatment of brain tumors[7,8].
Virus-originated HSV-TK gene is different from that of mammals, its product thymidine kinase is able to metabolize the nontoxic prodrug, GCV, into a monophosphate derivative, then phosphorylate it further into GCV triphosphate. This metabolite is incorporated into replicating DNA strands and acts as both a DNA synthesis inhibitor and a cell cycle blocker, finally leading to cell apoptosis and death[9,10], which is also called “suicide gene”. The therapeutic effect of this system is also based on a “bystander effect” whereby HSV-TK gene modified tumor cells are toxic to nearby unmodified tumor cells when exposed to the antiviral drug GCV. In this study, we wanted to see whether suicide gene therapy system was effective for the treatment of human pancreatic cancer.
Murine fibroblasts NIH3T3 cells and packaging cell PA317 were propagated in DMEM (Gibico) with low and high concentrations of glucose separately supplemented with 10% heat-inactivated fetal bovine serum (FBS). Human pancreatic cancer cell line SW1990 was maintained in RPMI1640 (Gibico) supplemented with 20% FBS. Transgenic cell PA317/TK and SW1990/TK were maintained in DMEM (10%FBS) and RPMI1640 (20% FBS) respectively, both with 300 μg/ml of G418 (Promega).
HSV-TK gene was inserted downstream of the cytomegalovirus (CMV) promoter in a GINa plasmid containing neo open reading frame (NeoORF), the neomycin resistance gene, which is resistant to neomycin analogue G418. Packaging cells PA317 were transfected with a GINaTK plasmid vector (a gift from the Genetic Research Center of Fudan University, Shanghai, China) using Lipofectin as recommended by the manufacturer (Gibico). Transfected cells were selected with 300 μg/ml of G418 in DMEM supplemented with 10% FBS for weeks. Single-clones were selected, expanded, and maintained in G418-containing medium until further experiments. Total cell DNA were extracted for PCR using specific primer sets for HSV-TK gene (Prime 1: 609 bp - 630 bp: 5’-CTACACCACACAACACCGCCTC-3’; Prime 2: 1012 bp - 991 bp: 5’-TCGCAGCCAGCATAGCCAGGTC-3’). We also used scanning-electronmicroscope (SEM) to confirm the success of gene transfer and the production of viral progeny. Virus titers were determined on NIH3T3 cells by plague assay.
One day before transfection, we seeded cells to be infected with a density that would allow them to grow logarithmically for at least 2-3 days. The transfection protocol was that the culture supernatant of PA317/TK containing progeny virion was collected and then replaced by fresh DMEM (10% FBS, without G418) within 24 hours before transfection, and passed through a 0.22 μm filter. Then the cell growth medium of SW1990 was replaced with 5 ml of viral supernatant containing 8 μg/ml polybrene to help the adhesion of viruses. Cells were returned to the incubator and incubated for 2 hours, and then 5 ml of growth medium was added and incubated overnight. The next day the supernatant was removed and the cells were fed with. Two days post-infection the selection was started with 300 μg/ml of G418 in RPMI1640. In order to confirm the successful infection we performed PCR as described before. We also extracted total RNA and did RT-PCR to assess the expression of TK gene.
We planted gene-modified pancreatic cell SW1990/TK in 96-well (Nunc) plates with 5000 cells/well. The next day, GCV was added at a concentration ranging from 0 to 500 μg/ml. five days later, cell survival rate (SR) was determined using routine MTT-method, absorbance (A) values were read on a Bio-Rad micro-plate auto-reader at 490 nm wavelength. Survival percentage was determined by ratios of absorbance values from test conditions over absorbance values from non-infected cells. SR = (A value of the test well ÷ A value of the control) × 100%.
SW1990 and SW1990/TK cells were seeded at a total density of 10000 cells/well in 96-well plates with various proportions. GCV was added at a concentration of 50 μg/ml. Then MTT assay was performed as routine protocols, each ratio was tested at least three times.
Uni-variate two-sided analysis of matched t-test for dependent samples was used, the dose-response curve was obtained using Microsoft Excel.
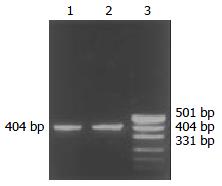
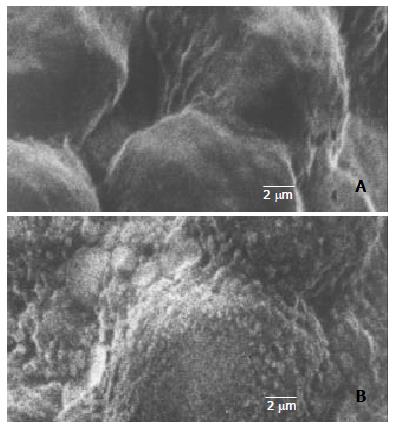
Through PCR, we obtained a 404 bp long fragment, which was the expected product of HSV-TK gene (Figure 1). From SEM, we can see easily that the cell surface of transfected PA317 had a lot of progeny viruses compared with their parent cells (Figure 2). These confirmed that TK gene was successfully integrated into the genome of packaging cell PA317, which was secreting virion progeny steadily, so we named it as PA317/TK. The virus titer was 40000 CFU/ml.
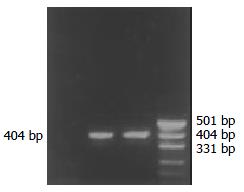
Both PCR and RT-PCR confirmed the successful infection and expression of TK gene in gene-modified SW1990 (RT-PCR product electrophoretogram see Figure 3), and we named this cell as SW1990/TK.
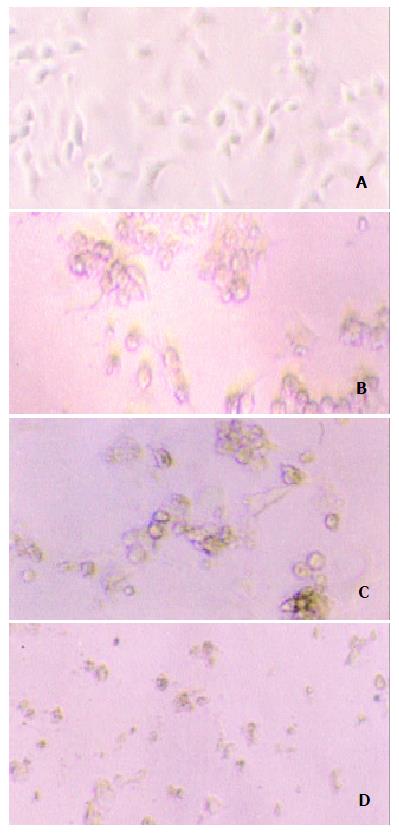
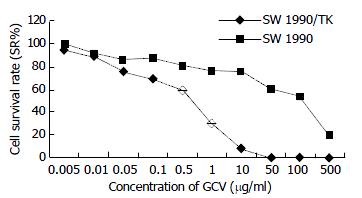
Our experiment demonstrated that transgenic SW1990/TK cells were sensitive to prodrug GCV compared to SW1990 (t = 4.15, n = 10, P < 0.0025). With increase of GCV concentration, SW1990/TK presented typical morphological changes of apoptosis and cell death such as nuclear condensation and oligonucleosomal DNA fragmentation, and finally lyses (Figure 4). The survival rate decreased sharply, especially at 0.5 to 50 μg/ml GCV, while the growth of SW1990 cells was not affected. At the same time, we found that the growth of unmodified cancer cells was inhibited when the concentration was more than 100 μg/ml, as seen in Figure 5.
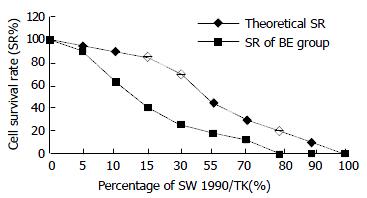
SW1990/TK exhibited a “bystander effect” when mixed with TK-negative cells at different ratios. From Figure 6 we can see that the cell survival rate was 40%, 20% and 0% when there was 15%, 30% and 80% of SW1990/TK cells in all, the inhibitory rate (IR = 1-SR) was 60%, 80%, 100% respectively. Apparently the IR was much higher than the percentage of SW1990/TK, which reflected the bystander effect.
Pancreatic cancer has an extremely poor prognosis due to lack of early diagnostic and therapeutic approaches, few are suitable for surgery and respond to chemo-radiation therapy, mainly because of its silent course and explosive fatal outcome. Most patients had locally advanced or metastatic diseases at the time of diagnosis and were therefore not amenable to resection, whilst chemotherapy and radiotherapy were by and large ineffective[11]. HSV-TK/GCV suicide gene therapy system has been widely studied these years, and is a promising approach to tumor therapy, but its use in human pancreatic cancer has been limited.
In our study, the retrovirus was used as a target gene vector. Among various vectors, retrovirus-mediated gene transfer is restricted to cells that are proliferating and synthesizing DNA at the time of infection but not nondividing cells. It is suitable to gene transfer of malignant cells with rapid proliferation and can improve the targeting of gene transfer. With 40000 CFU/ml virus titer, it transfers target gene effectively.
Besides the advantage of retrovirus, HSV-TK/GCV suicide gene therapy system shows another superiority: bystander effect. Both the transduction efficiency and bystander effect are essential factors for the success of the anti-tumor effect of HSV-TK and prodrug GCV suicide gene therapy system. Bystander effect is described when nontransduced or genetically unmodified cells are killed as the result of enzyme-prodrug activation during the death of genetically modified tumor cells transduced with a suicide gene. The “bystander effect” greatly amplifies the efficacy of HSV-TK/GCV gene therapy for cancer in which only a fraction of the cells are targeted. The in vitro bystander effect in C6/C6-TK5 co-culture was highly significant, the presence of only 5% of C6-TK5 cells led to an overall 78% decrease in cell survival after 5 days of GCV treatment[12]. In our experiment, 15% of SW1990/TK cells could lead 60% cells to death. However, the mechanism of bystander effect is still controversial. Some investigators suggested that intercellular communication was essential for the bystander effect. The correlation between gap junction communication (GJIC) and the extent of bystander effect suggested a role of GJIC in mediating the bystander effect, which could provide a useful system for selective killing of gene-modified tumor cells[13]. Some provided evidences for a role of cell membrane in signal pathways leading to bystander effect.
As we all know, everything has two sides. Inevitably, this system had its own side effect. The most common side effects of GCV in immunocompromised patients were leukemia (7% - 42%), thrombocytopenia (8% - 57%), and abnormal liver-function tests (2%). However, side effects in patients with intact immune system have not been reported[14]. As we can see in Figure 5, once the dose of GCV exceeded 500 μg/ml, it did harm to unmodified cells, so control of the dosage of drug administration is important in clinical practice.
The current trend of gene therapy for tumor is to combine two or even more approaches in order to improve its anti-tumor effect and reduce its side effects. The combination of both suicide systems of cytochrome p450 2b1 (CYP2B1)/CPA and HSV-TK/GCV in vitro resulted in a potentiation of the killing effect. This suggested that in order to achieve a potentiation in cell killing when two suicide systems were combined, co-expression of both genes in the same tumor cell would be necessary[15]. Gene therapy with p53 and K-ras modulated herpes viruses might become a palliative treatment option and could be used easily by regional chemotherapy techniques[1]. Pancreatic ductal adenocarcinomas (PDACs) could overexpress various cell-surface tyrosine kinase receptors, including type I high-affinity fibroblast growth factor receptor (FGFR-1). In view of the overexpression of high-affinity FGFRs in cancer cells in PDAC, Kleeff’s findings suggested that combined use of AdTK, ganciclovir, and FGF2-Fab’ might ultimately be a promising therapeutic approach in a subgroup of patients with PDAC[16].
Besides suicide gene therapy system, there are many other approaches for pancreatic cancer therapy. Based on the relative uniformity in its molecular abnormalities, about 80% - 90% of tumors have dominant oncogene K-ras mutations and disruption of p16/RB tumor suppressor pathway, about 60% - 75% have p53 mutations, and more than 50% have SMAD4/DPC4 disruptions, transfer of wild-type p53 and p16 could produce significant growth suppression of pancreatic cancer in vitro and in vivo [17-19]. The results of Kawakami et al[20] showed that IL-4 receptor-targeted cytotoxin represented a potent agent that might provide an effective therapy for pancreatic cancer. Yan and his group found that adenovirus-mediated E2F-1 gene transfer could sensitize melanoma cells to some chemotherapeutic agents, particularly topoisomerase II poisons in vitro and in vivo. These results suggested a new chemosensitization strategy for melanoma gene therapy[21]. With the discovery of RNA-interference, some researchers suggested it might be used in tumor therapy[22-24].
Our results indicated that gene modified tumor cells SW1990/TK were sensitive to antivirus prodrug GCV, and showed a remarkable bystander effect on killing tumor cells. In conclusion, HSV-TK/GCV suicide gene therapy system is a promising approach for the treatment of pancreatic cancer. As increasingly more researchers focus on the diagnosis and therapy of this lethal malignancy, we believe that some effective ways would be discovered for clinical treatment of pancreatic cancer.
First of all, I thank my family and my boyfriend for offering their all-out support to my work. Secondly, I appreciate the academic advice and technical support by Professor Zhang LS and Mrs. Lu.
Edited by Zhu LH and Wang XL
| 1. | Lorenz M, Heinrich S. Regional chemotherapy. Hematol Oncol Clin North Am. 2002;16:199-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Gustin A, Pederson L, Miller R, Chan C, Vickers SM. Application of molecular biology studies to gene therapy treatment strategies. World J Surg. 2002;26:854-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Shankar A, Russell RC. Recent advances in the surgical treatment of pancreatic cancer. World J Gastroenterol. 2001;7:622-626. [PubMed] |
| 4. | McAuliffe PF, Jarnagin WR, Johnson P, Delman KA, Federoff H, Fong Y. Effective treatment of pancreatic tumors with two multimutated herpes simplex oncolytic viruses. J Gastrointest Surg. 2000;4:580-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 5. | Mäkinen K, Loimas S, Wahlfors J, Alhava E, Jänne J. Evaluation of herpes simplex thymidine kinase mediated gene therapy in experimental pancreatic cancer. J Gene Med. 2000;2:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Xu CT, Huang LT, Pan BR. Current gene therapy for stomach carcinoma. World J Gastroenterol. 2001;7:752-759. [PubMed] |
| 7. | Fukui T, Hayashi Y, Kagami H, Yamamoto N, Fukuhara H, Tohnai I, Ueda M, Mizuno M, Yoshida J. Suicide gene therapy for human oral squamous cell carcinoma cell lines with adeno-associated virus vector. Oral Oncol. 2001;37:211-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Jiang BJ, Sun RX, Lin H, Gao YF. Study on the risk factors of lymphatic metastasis and the indications of less in vasive operations in early gastric cancer. World J Gastroenterol. 2000;6:553-556. [PubMed] |
| 9. | Robe PA, Princen F, Martin D, Malgrange B, Stevenaert A, Moonen G, Gielen J, Merville M, Bours V. Pharmacological modu-lation of the bystander effect in the herpes simplex virus thymi-dine kinase/ganciclovir gene therapy system: effects of dibutyryl adenosine 3', 5'-cyclic monophosphate, alpha-glycyrrhetinic acid, and cytosine arabinoside. Biochem Pharmacol. 2000;60:241-249. [RCA] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Craperi D, Vicat JM, Nissou MF, Mathieu J, Baudier J, Benabid AL, Verna JM. Increased bax expression is associated with cell death induced by ganciclovir in a herpes thymidine kinase gene-expressing glioma cell line. Hum Gene Ther. 1999;10:679-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Halloran CM, Ghaneh P, Neoptolemos JP, Costello E. Gene therapy for pancreatic cancer--current and prospective strategies. Surg Oncol. 2000;9:181-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Gilliam AD, Watson SA. Emerging biological therapies for pancreatic carcinoma. Eur J Surg Oncol. 2002;28:370-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Nagasawa H, Cremesti A, Kolesnick R, Fuks Z, Little JB. Involvement of membrane signaling in the bystander effect in irradiated cells. Cancer Res. 2002;62:2531-2534. [PubMed] |
| 14. | Shalev M, Miles BJ, Thompson TC, Ayala G, Butler EB, Aguilar-Cordova E, Kadmon D. Suicide gene therapy for prostate cancer using a replication-deficient adenovirus containing the herpesvirus thymidine kinase gene. World J Urol. 2000;18:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Carrió M, Visa J, Cascante A, Estivill X, Fillat C. Intratumoral activation of cyclophosphamide by retroviral transfer of the cytochrome P450 2B1 in a pancreatic tumor model. Combination with the HSVtk/GCV system. J Gene Med. 2002;4:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Kleeff J, Fukahi K, Lopez ME, Friess H, Büchler MW, Sosnowski BA, Korc M. Targeting of suicide gene delivery in pancreatic cancer cells via FGF receptors. Cancer Gene Ther. 2002;9:522-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Gazdar AF, Minna JD. Targeted therapies for killing tumor cells. Proc Natl Acad Sci USA. 2001;98:10028-10030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Ghaneh P, Greenhalf W, Humphreys M, Wilson D, Zumstein L, Lemoine NR, Neoptolemos JP. Adenovirus-mediated transfer of p53 and p16(INK4a) results in pancreatic cancer regression in vitro and in vivo. Gene Ther. 2001;8:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Zheng M, Liu LX, Zhu AL, Qi SY, Jiang HC, Xiao ZY. K-ras gene mutation in the diagnosis of ultrasound guided fine-needle biopsy of pancreatic masses. World J Gastroenterol. 2003;9:188-191. [PubMed] |
| 20. | Kawakami K, Kawakami M, Husain SR, Puri RK. Targeting interleukin-4 receptors for effective pancreatic cancer therapy. Cancer Res. 2002;62:3575-3580. [PubMed] |
| 21. | Dong YB, Yang HL, Elliott MJ, McMasters KM. Adenovirus-mediated E2F-1 gene transfer sensitizes melanoma cells to apoptosis induced by topoisomerase II inhibitors. Cancer Res. 2002;62:1776-1783. [PubMed] |
| 22. | Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3486] [Cited by in RCA: 3462] [Article Influence: 150.5] [Reference Citation Analysis (0)] |
| 23. | Paddison PJ, Caudy AA, Hannon GJ. Stable suppression of gene expression by RNAi in mammalian cells. Proc Natl Acad Sci USA. 2002;99:1443-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 399] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 24. | Borkhardt A. Blocking oncogenes in malignant cells by RNA interference--new hope for a highly specific cancer treatment. Cancer Cell. 2002;2:167-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |