INTRODUCTION
Angiogenesis is the process of sprouting of capillaries form preexisting blood vessels. The overall process is a complex one that involves many biological functions and cell types. Previous researches since 1970s have demonstrated that tumor angiogenesis is required for the growth and metastasis of primary solid tumor. Endothelial cell activation, migration, and proliferation are major cellular events in this process. All of these processes are under the tight regulation of factors that either “promote” or “inhibit” angiogenesis. When the balance of these factors is disturbed, angiogenic factors can be released from tumor cells, such as vascular endothelial growth factor (VEGF)[1] and basic fibroblast growth factor (bFGF), and migrate to the nearby blood vessel endothelia, signal the activation of the angiogenic response. Tumor cells also produce anti-angiogenic factors including angiostatin, endostatin. Administration of endostatin in tumor-bear mice has been shown to keep the primary tumor in a dormant state[2]. But the mechanisms are still unclear. One mechanism is that endostatin incombination with the endothelial cells to form a compound with tyrosine activity and thereby inducing apoptosis of endothelial cells. The other is that endostatin functions as a ligand for the integrin family of adhesion receptors on the surface of endothelial cells and is associated with MMP family[3]. So, in order to explore the mechanism of endostatin, we provided data that endostatin showed a broad antitumor effect, which might be a result of its inhibitory mechanism on tumor angiogenesis.
MATERIALS AND METHODS
Materials
Endostatin donated from Hefei Sunny Biology-technology Institute, was a 20 ku C-terminal fragment of collagen X VIII. Human endostatin was cloned and expressed in Escherichia coli and purified by gene cloning technology by Gene Med Ltd.
Colon cancer cell line HL-174T was purchased from Shanghai Cellular Research Institute. The cells were cultured in 1640 medium supplemented with 100 mL/L fetal bovine serum at 37 °C in 50 mL/L CO2 and the culture media were changed twice a week and observed in the invert microscope to assay the reproduction situation. All reagents and media for cell culture were obtained from GIBCO.
Methods
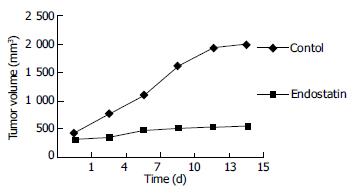
Subcutaneous xenograft models Tumor cells were implanted (0.5 × 106 cells/animal) subcutaneously using a 27-gauge needle in the back region of BALB/C-nu/nu female mice of 6-8 wk old. Tumor volume was measured every 3 d using a slide gaug. Tumor volumes were measured as the product of (length × width) × П/6. Mice were fed with a standard rodent diet for two weeks, and 14 of 20 mice with a similar tumor volume were selected and divided into two groups randomly. Animals of each group were treated once daily with a 0.1mL (2 mg/mL) ip bolus injection of endostatin in saline or saline alone for 14 d. Upon termination of the efficacy portion of endostatin (3 d after drug withdrawal), the animals were euthanized, and the tumor was harvested from the animals and the volume was weighted. The tumors were submitted for immunohistochemical test.
Evaluation of MVD, VEGF and VEGFR/flk-1 expression in tumors Five-μm paraffin embedded sections of the tumors were evaluated by immunohistochemistry. The sections were stained with anti- CD34 antibody (DAKO, 1:2000) and polyclonal anti-VEGF antibody and polyclonal anti-Flk-1 antibody that could recognize VEGF isoforms and their receptors, following avidin-biotin complex methods.
Microvessel density was assessed by light microscopy in areas of invasive tumors containing the highest number of capillaries and small venules per area (neovascular “hot point”). Areas of the highest neovascularization were found by scanning the tumor sections at a low magnification (× 100) and identified with the greatest number of distinct CD34 stained (brown) microvessels per area. Necrotic areas within tumors, where microvessels were sparse, were not included in the vessel counts. After the area of the highest neovascularization was identified, individual microvessels were counted under × 400 field. A brown-stained endothelial cell or a endothelial cell cluster clearly separated from adjacent microvessels, tumor cells, and other connective-tissue elements, considered a single, countable microvessel. Vessel lumens were not necessary for a structure to be defined as a microvessel, and red cells were not used to define a vessel lumen. Results were expressed as the number of microvessels identified within 10 fields (× 400).
VEGF was assessed by light microscopy (× 100) in the areas of invasive tumor cells. VEGF expression was determined on adjacent serial visual fields and scored by a pathologic doctor. The number of positively and negatively stained cells was counted in 10 continuous visual fields. If more than 5% cells were brown stained, they were considered as positive, otherwise negative. The results were expressed as mean ± SD.
Flk-1 was assessed by light microscopy (× 400) in the areas of microvessel endothelial cells. The number of endothelial cells stained positively was counted in 10 continuous visual fields. Over 5% positively stained endothelial cells were determined as positive and else negative. The results were expressed as mean ± SD.
Statistics
The analysis of variance test was used to compare the differences in tumor weight and MVD between the treated and control groups. The Chi-squared test was used to compare the differences in the expression of VEGF or VEGFR2/Flk-1 between the treated and control groups. The difference was considered statistically significant if the P value was less than 0.05. All the data were analyzed with SAS 6.22 by the statistical office of the Second Military Medical University.
DISCUSSION
Angiogenesis is a fundamental process by which new blood vessels are formed. It is essential in reproduction, development, and wound repair. Under these conditions, angiogenesis is highly regulated, turned on for brief periods and then completely inhibited. However some diseases are driven by persistently unregulated angiogenesis. Tumor growth is one of these kinds of diseases. Tumor growth and metastasis are angiogenesis-dependent. A tumor must continuously stimulate the growth of new capillary blood vessels for the tumor itself to grow. MVD was significantly high in colon cancer tissues compared with normal colon tissues[4]. Tumor cells get oxygen and nutrition through angiogenesis. The Phenomena were also observed in other cancers, for example, breast carcinoma[5] lung cancer[6] prostate cancer[7] and pancreatic tumor[8], etc.
Endostatin, a 20-kD C-terminal cleavage product of collagen XVIII, was originally identified by O’Reilly et al[2] as a tumor-derived, highly active, and endothelial specific angiogenic inhibitor[9]. Recombinant endostatin has been shown to inhibit the growth of a wide variety of tumors in mice, with no toxic side effects observed. Importantly, tumors treated with several cycles of endostatin did not develop drug resistance and become dormant, which persisted even when the endostatin therapy was discontinued[10]. In our experiment, we found that the tumor growth slowed down or even stopped three days after usage of endostatin, but when the treatment was stopped the tumor began to grow rapidly. So endostatin must be used continuously to prevent tumor growth. It is difficult for the patients to accept the treatment in their whole lifetime, so further research is necessary for the usage of endostatin. At present, the molecular mechanism of endostatin action remains unknown. There are some theories which are supported by various studies.
Our results showed that endostatin blocked the VEGF-VEGFR/Flk-1 pathway to inhibit the angiogenesis of colon tumor in nude mice. VEGF pathway is the only well-defined signaling pathway known to be required for the normal development of vasculature as well as for the pathologic angiogenesis that accompanies cancer and other disease states[1,11,12]. The VEGF family of growth factors comprises at least five members, i.e., VEGF, placenta growth factor (PGF) , VEGF-B , VEGF-C[13], and VEGF-D. The VEGF pathway is initiated when VEGF binds to its receptors in endothelial cells. The three best characterized VEGF receptors are termed VEGF receptor 1 (VEGFR1/Flt-1) and VEGF receptor 2 (VEGFR2/Flk-1/KDR) and VEGF receptor 3 (VEGFR3/Flk-4). VEGFR1 and VEGFR2 are highly related transmembrane tyrosine kinases that use their ectodomains to bind to VEGF[14,15]. These binding in turn, activate the intrinsic tyrosine kinase activity of their cytodomains, initiating intracellular signaling. VEGFR3 is expressed almost exclusively by the lymphatic endothelium, thus being considered as a major regulator of lymphangiogenesis[16]. The blockade of VEGF by using a soluble VEGFR-3 extracellular domain could inhibit tumor lymphangiogenesis[17]. While PIGF could bind selectively to VEGFR1, VEGF-C and VEGF-D could bind to both VEGFR3[18,19] and VEGFR2. The corresponding receptor(s) for VEGF-B is VEGFR1. VEGF/VEGFR2 pathway is the most important for tumor angiogenesis, growth and metastasis. In our studies, we found VEGFR2 was obviously down-regulated in the endostatin group compared to the control group, but the expression of VEGF was not changed in both groups. So we think that endostatin can block the VEGF/VEGFR2 pathway by binding to VEGFR2, thus inhibiting the neovascularization.
Endostatin could function as a ligand for the integrin family of adhesion receptors on the surface of endothelial cells[20]. Integrins are potential targets for endostatin function and they are important in endothelial cell biology and angiogenesis. The endostatin-integrin interaction is of functional significance in vitro, and the immobilized endostatin supports endothelial cell survival and migration in an integrin-dependent manner. Soluble endostatin in turn could inhibit integrin-dependent endothelial cell functions, such as cell migration[20].
Matrix metalloproteinases (MMPs), a family of extracellular and membrane-associated endopeptidases, collectively digest almost all extracellular matrix and basement membrane components, thus playing an important role in tumor progression. Endostatin could prevent the fragmentation of pro-MMP-2 that is associated with reduction of catalytic activity. Endostatin had no effect on MMP-8 as shown by collagenase activity assays[3]. Endostatin could block the activation and activities of certain tumor-associated pro-MMPs, such as pro-MMP-2, -9, and -13, which may explain at least in part, the antitumor effect of endostatin[3,21].
Inhibition of angiogenesis has been shown to be an effective strategy in cancer therapy in mice. However, its widespread application has been hampered by difficulties in the large-scale production of antiangiogenic proteins. This limitation may be resolved by in vivo delivery and expression of antiangiogenic genes. A recombinant adenovirus that could expresses murine endostatin that was biologically active in vitro as determined in endothelial cell proliferation assays, and in vivo by suppression of angiogenesis induced by VEGF has been constructed[22]. Persistent high serum levels of endostatin were achieved after systemic administration of the vector to nude mice, which resulted in a significant reduction of the growth rates and the volumes of metastatic brain tumor[23]. If the method can be used safely in clinic, it can effectively resolve the recurrence and overgrowth of tumors during the rest periods. Different targets have been found and effective results were achieved in gene therapy. An adenoviral vector carrying Tie2 gene, an endostatin-specific receptor tyrosine kinase, was constructed and tested in established primary tumor, a murine mammary carcinoma or a murine melanoma, which could significantly inhibit the growth rate of both murine mammary carcinoma and melanoma by 64% and 47%, respectively. So the potential of vector-mediated antiangiogenic gene therapy as a component is a very novel and effective strategy in cancer therapy[24,25].
Synergy between endostatin and chemotherapy[26] can be used to eradicate spontaneous tumor and metastases of colon cancer or other angiogenesis dependent diseases. Tumor necrosis was demonstrated only in animals receiving the combination therapy, but not when each agent was applied as monotherapy. The results suggested that these synergistic treatment modalities might provide a novel and effective tool for future therapies of metastatic cancer. Synergy between endostatin and interventional therapy is another way to inhibit the neovascularization. Endostatin can be sent directly to the microvessels around the tumor and destroy the tunica intima of vessels to starve the tumor to death. Other strategies also were used to block the VEGF/VEGFR pathway to inhibit the tumor growth. VEGF-trap is a potent blocker against the angiogenesis of the tumor and could result in stunted and almost completely avascular tumors. VEGF-Trap-mediated blockade may be a hopeful way for tumor treatment[27]. More and more attention has been paid to the effectiveness and carrier and medication methods are paid more and more attention throughout the world. Endostatin may provide a novel and effective tool for future therapies of cancer[28,29].