INTRODUCTION
Previous microscopic anatomy studies have shown that somatic and visceral nerves are both widely approached to MC. MC and nerve cells interact with each other by connecting with a lemma or degranular style, so that they could regulate microenvironments. As far as organizations are concerned, the anatomic relation means interaction with function, but no studies are available about the development and distribution of MC and developmental regularities of SP, CGRP-IR nerves and cells in human fetus duodenum. BY investigating the relationship between MC and nerve- endocrine- immunological network[1-3], we observed the histological changes in human fetus duodenum with HE staining, the developmental regularities and heterogeneity of MC with TB special staining, neuropeptide SP, CGRP by ABC methods, the relations between neuropeptide and MC. The study provided morphology data of the functional significance of mast cells in human fetus duodenum.
MATERIALS AND METHODS
Tissue specimens
Twenty-one fetuses of 3-9 mo old and one dead term infant were randomly collected within 1-5 h after birth, in which 10 were males and 11 were females. Duodena were taken out (near to bulbs), and fixed in 40 g/L formaldehyde for 12 h, cut into 10 mm × 5 mm × 3 mm, then embedded in paraffin and cut into 5 μm thick serial section.
TB special staining
The paraffin embedded sections were deparaffined in serial xylene, dehydrated by alcohol solvents and mounted by xylene transparent neutral gum, then examined by microscopy and photographed.
Immunohistochemistry
The reactions were carried out according to the ABC method as previously reported[4]. Briefly, the paraffin sections were deparaffined in xylene and graded alcohol. Sections were incubated at room temperature for 10 min with 30 mL/L H2O2 solution to block endogenous peroxidase activity. After washed with phosphate buffered saline (PBS) 3 times for 5 min each, slides were digested with trypsin, treated with 3 g/L Triton X-100, followed by incubation with antibodies SP (1:2000, Sigma) or CGRP (1:1000, Sigma) at 37 °C for 2 h and at 4 °C for 24 h. The sections were then incubated with biotin-conjugated IgG (diluted in PBS, 1:100) for 2.5 h at room temperature and washed with PBS 3 times for 5 min each, followed by incubation with the streptavidin-peroxidase complex for 1 h. At last, chromogen 3,3’-diaminobenzidine tetrahydrochloride (DAB) (1:50, Wuhan Boster) was added to visualize the reaction products of peroxidase.
The specific neuropeptide antibodies were replaced by PBS or normal rabbit serum for the negative controls. Adult duodenum tissue sections were used as positive controls, which showed immunoreactivity for SP and CGRP.
The results were judged as follows. Pale-yellow was negative (-), shallow brown was weak positive (+), brown was moderately positive (++), and deep brown was strongly positive (+++).
RESULTS
HE staining
The histological differentiations were found in lobe-shape intestinal villi in duodenum at the 12nd wk. At the 15th wk, duodenal glands of mucous cells were formed in the submucosa. At the 21st wk, muscular mucosa appeared with 4 layers in the wall of duodenum gradually.
TB special staining
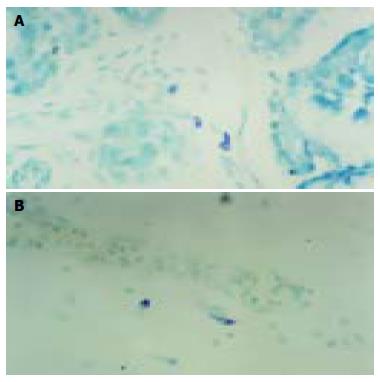
The experiments showed that CTMC in submucosa and muscular layer appeared by staining with 5 g/L toluidine blue with 500 mL/L alcohol dyeing for 5 min, but MMC in mucous layer appeared by staining with 5 g/L toluidine blue with one equivalent hydrochloric acid for 5 d. At the 16th wk, CTMC appeared occasionally in submoucosa. But the time of MMC appearance was at the 18th wk. The granules in immature MC were pale violet, and strong violet in the mature MC as detected by TB special staining. CTMC in intermuscular appeared fusiform and cell bodies were small. MC in mucosa and submucosa layer were round or oval in shape and cell bodies were large (Figure 1A). With the increase of gestational age, the number of MC in duodenal wall increased gradually. At the 22nd wk, the two types of MC were activated and distributed in the surrounding blood vessels and ganglions. The verge of some MC was unclear, which might be a degranular phenomenon (Figure 1B). MC were closed together with connective tissue cells and there existed lemma connection in fetus duodenal mucous and submucous layer connective tissues.
Figure 1 MC in submucous layer at the 21st wk (A) and in mucous layer at the 27th wk (B) of duodenum (TB × 400).
Immune phenotype
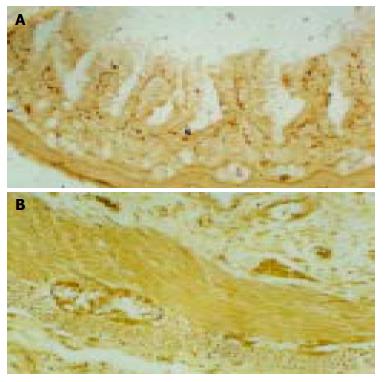
The degree of positive SP-immunoreactivity (SP-IR) and CGRP-immunoreactivity (CGRP-IR) and their distribution in neurons and nerve fibers (NF) in fetus duodenum had no sex difference. At the 14th wk, SP-IR positive neurons appeared occasionally in the myenteric plexuses of duodenum and neuron cell bodies showed fusiform, few SP-IR-NF appeared in submucosal layer. But nerve plexuses of CGRP-IR appeared at the 16th wk. Diffused SP and CGRP-IR neurons were appeared in submucous at the 21st wk, pale-brown neuron cell bodies were round or oval in shape and nuclei were negative (Figure 2B). SP-IR-NF response to reinforcement (+ to ++) was found in myenteric connective tissue, showing string pearls in shape. A few CGRP-IR-NF appeared in submucous (+), were showing point line in shape. With the growth of gestational age, nerve fibers were mainly distributed in connective tissue of mucous, submucous and myenteric layers. Nerve plexus density increased, and staining turned deep gradually. The dark black positive nerve plexuses were identified (+++) in fetus duodenum after 34th wk, showing network structures and obvious varicosity (Figure 2B). On the corresponding site of serial sections, SP-IR and CGRP-IR materials were partially coexisted in nerve fibers and neurons. In serial sections of TB histochemistry and ABC immunohistochemistry staining, about 10% MC were SP-IR positive, and less MC were CGRP-IR positive. Some of SP, CGRP-IR-NF positive cells were coexisted with MC, while some were closely contacted.
Figure 2 CGRP-IR (+) nerve fiber in duodenum submucous layer at the 21st wk (A) (ABC × 100) and SP-IR (++) neuron in duodenum submucous layer and myenteric nerve plexuses at the 28th wk (B) (ABC × 200).
DISCUSSION
The results of our experiment showed that there were two kinds of MC in connective tissue of fetus duodenum, namely MMC and CTMC, which possessed heterogeneity. our results are consistent with Wang et al[5]. It might be related to many factors such as local microenvironment. The activated MC were localized around cells, small vessels and ganglions in submucosa, showing that MC were related with those cells and tissues. Surface of some MC boundary was not clear. MC showed degranulation, which means that the cells were releasing active media and affecting peripheral environments and cells, so we considered that MC might take part in allergic and other physiological activities during fetus development and maturation of its surrounding tissues and organs. With electron microscope, Li et al[6] observed MC cytoplasm in intestinal mucosa lamina propria had electron dense round granules and electron-lucent inequality of size vesicles, which were phenotype of activated MC. MC were important effector cells. It conducted the signal of inflammation cells, and induce the occurrence of mucosa correlated diseases[7-9]. Previous studies have shown the severity of gastrointestinal allergic diseases, such as Hp infection, stomach reflow in duodenum, neuro-psychical factor leading gastro-intestinal functional dyspepsia related with MC, and the more serious the disease, the more obviously increased the numbers of MC[10,11]. MC could release inflammatory medium, secretory cytokines (IL-2, TNF-α), biologically active molecules through phospholipase A2 pathway in earlier period of various kinds of enteritis, which could evoke physiological injuries in the intestinal[12-14]. With the growth of gestational age, the number of MC in bowel wall connective tissue increases gradually, suggesting that MC play an important regulatory role in developing tissue cells in duodenum.
Our experiment showed that the SP-IR and CGRP-IR peptidergic nerves plexus appeared at different time. Owing to the difference of local microenvironments in gastrointestinal tract, various kinds of peptidergic nerves appeared in different time. This difference was decided by local microenvironments of neural crest cells on the way of migration or after its presence. The occurrence of peptidergic nerves was determined by interaction of microenvironmental factors and crest cells. Since different investigators used different specimen source, they adopted different methods and techniques, the detection rate of positive nerve fibers and neurons was different. for example, Larsson et al[15] reported that CGRP-IR-NF was not observed in fetus duodenum mucosa membrane. But Yang et al[16] reported that developing fetus from the 13th week showed mild CGRP-IR-NF in myenteric nerve plexus of duodenum. There were differences in the distribution of SP and CGRP peptidergic nerve plexus at the same gestational age. We found that the distribution and response of SP-IR-NF was slightly higher than those of CGRP-IR-NF in myenteric nerve plexus, but CGRP-IR-NF mainly existed in connective tissue of mucosa and submucosa layers, showing that there was a difference in distribution of peptidergic nerve fibers in the same individual organ. They were closely connected with the presence of digestive tract peptidergic nerve. SP peptidergic nerves exist mainly in duodenum myenteric connective tissue. It could activate cholinergic nerve of gastrointestinal tract, cause neurilemma depolarization in myentenic nerve plexus and increase the effect of acetylcholine, resulting in contraction of digestive tract smooth muscle and secretion of glands[17]. CGRP peptidergic nerves exit mainly in mucous and submucous layers. It is probably related to differentiation of duodenal epithelium and excretion of duodenal gland. CGRP could expand blood vessels of gastrointestinal tract and increase capillary permeability. CGRP could inhibit smooth muscles of gastrointestinal tract and cause relaxation of circular and longitudinal muscles and decrease duodenum vermiculation[18]. Previous studies have reported that decrease of the level of serum CGRP might be related to Hp infection, leading to occurrence of duodenal ampulla ulcer[19]. By observing adjacent sections, we found there was partial concordance in localization of SP-IR and CGRP-IR positive neurons. Therefore it is possible that SP and CGRP neurotransmitters coexist in peptidergic nerves. In previous studies, besides the coexistence of CGRP, cells of SP, somatostatin, vasoactive intestinal peptide (VIP) and neuropeptide Y were also coexisted in thymus, sensory ganglion and other organs[18,20,21]. Because SP, CGRP peptidergic nerve fibers project very extensive in central nervous system, it is possible that these neuropeptides together with nervous system produce an important effect on sensation, movement, endocrine and other aspects. Gastrointestine SP,CGRP could regulate stability of MC, which might play an important role in keeping homeostasis the occurrence and development of many pathologic alternations.
There are a lot of MC in connective tissue of human duodenum. Meanwhile, there also existed a huge nervous system, including rich peptidergic neurotransmitters[22,23]. Those made it possible to connect adequate information between MC and nervous system. Gastrointestinal mucous MC are next to gastrointestinal nervous system. It provides double directions of channel between center nervous system and intestinal tract. Thus nervous system can influence physiological functions of gastrointestinal tract. MC could also stimulate intestinal neurons and smooth muscle cells by releasing various kinds of media[24,25]. By electron microscopy some researchers found lemma connection was existed between MC plasmalemma and axon of nonmyelinated nerve fibers in enteron mucosa, form “plywood structure” and synaptic connection[6,26]. As far as organ is concerned, that MC closely contacted with nerves in anatomy means interaction of function by releasing certain active substances, such as histamine, nitrogen monoxide etc, as transmitters; On the other hand, nerves could affect MC growth and development. Animal experiments confirmed that the number of MMC and CTMC could be increased by nerve growth factor (NGF), moreover, NGF receptors have been discovered on the surface of MC in rat belly[27]. It has been confirmed that SP and VIP are coexisted in MC[28]. Jia et al[29-31] observed MC in mouse submandibular glands or tongue, and found that most of the MC were SP-IR positive, and 14% MC were CGRP-IR positive. In our studies, we observed that about 10% MC in the wall of fetus duodenum were SP-IR positive, but fewer MC were CGRP-IR positive, indicating that MC in different species and organs contain different peptidergic activity substances. Thus, MC in fetus duodenum possess heterogeneity, whose physiological significance needs further research.