INTRODUCTION
Liver fluke infestation caused by Opisthorchisviverrini remains a major public health concern in Southeast Asia[1], particularly in Thailand where an estimated 6 million people are infected[2]. This infection is associated with a number of benign hepatobiliary diseases, including cholangitis, obstructive jaundice, hepatomegaly, cholecystitis and biliary lithiasis[3]. Both experimental and epidemiological evidence implicate liver fluke infestation in the etiology of bile duct cancer, i.e. cholangiocarcinoma[1,4,5].
An association between liver fluke infection and biliary lithiasis is well-recognized. Opisthorchis worm and/or eggs have been observed in the stones of infected or previously treated individuals[6], just as Clonorchis sinensis eggs were found in the stones of those with Chinese liver fluke infection[7,8]. Several community-based studies in Northeast Thailand have shown a significant increase in the frequency of biliary sludge in people with a heavy infection[9-11]. Since biliary sludge is a precursor of stone formation[12-14], opisthorchiasis likely plays a role in the development of biliary stones in certain individuals. However, the mechanism of liver-fluke-associated stone development is unclear.
We report the incidence of stone types, its relationship with O.viverrini infection and bile/stone examinations. The potential pathogenesis of opisthorchiasis-associated stones, in patients from endemic areas of Thailand, is proposed.
MATERIALS AND METHODS
Patients
This study was carried out in Northeast Thailand, an area endemic for opisthorchiasis. The study population included 113 consecutive patients scheduled for cholecystectomy at Khon Kaen Regional Hospital from whom bile was obtained between 1990 and 1991. The gallbladder diseases associated with the cholecystectomies included cholelithiasis (n = 71), cholecystitis with cholangiocarcinoma (n = 29) and cholecystitis with other miscellaneous diseases (n = 13). None of the patients had any history of hemolytic anemia or severe thalassemia.
Patients ranged between 17 and 94 years of age (mean, 54.97 ± 14.65) (45 females, 68 males). The study was carried out in accordance with the principles embodied in the 1975 Helsinki Declaration. A signed informed consent was obtained from each patient.
Specimen collection and laboratory investigations
Gallstones and/or common bile duct stones and bile specimens were collected from each individual. Bile samples aspirated from the gallbladder were centrifuged at 3000 r/min for 10 min, then examined for the presence of O.viverrini eggs. At least four pellet-smears per individual were examined before the specimen was considered negative for eggs.
For a demonstration of calcium and bilirubin, the bile smears were air-dried and fixed in 40 g/L formaldehyde. Calcium and bilirubin staining were performed using histochemical methods[15]. Calcium was stained black, bilirubin deep green.
Five stones from each group with Opisthorchis egg-negative and -positive bile underwent scanning electron microscopic (SEM) study. Briefly, the stones were thoroughly washed in distilled water and dried. After mechanical breaking, the stones were sputter-coated with gold (agar aids-PS3, UK) and observed under a Hitachi (Model S-3200N, Japan) scanning electron microscope and photographed.
Statistical analysis
The χ2-test was used to analyze the association between the frequency of gallstones and sex, disease association, parasite egg status and age groups. A P value < 0.05 was considered statistically significant.
RESULTS
Cholesterol or pigment stones were classified by visual inspection. Cholesterol stones were white or yellow and crystalline in composition, pigment stones were black or black-brown and amorphous.
Of the 113 cases, 83 had stones while 30 had none. Most of the stone cases (98.8%, 82/83) had pigment stones, while only one had cholesterol stone(s). In addition, 63 of the 83 cases (75.9%) contained multiple stones, while the remainder had a single stone. Of the 29 cases with cholangiocarcinoma, 9 (31.0%) contained stones while the others with miscellaneous diseases had stones in 3 out of 13 (23.1%) cases. The prevalence of stones in all of the sample groups was significantly higher in females (χ2-test, P = 0.028). No association between stones and age groups (stratified as < 40, 40-49, 50-59 and > 60 years) was found (χ2-test, P≥ 0.05).
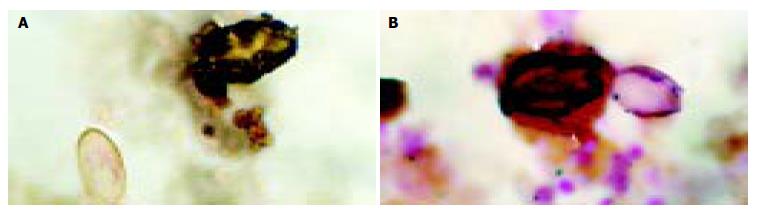
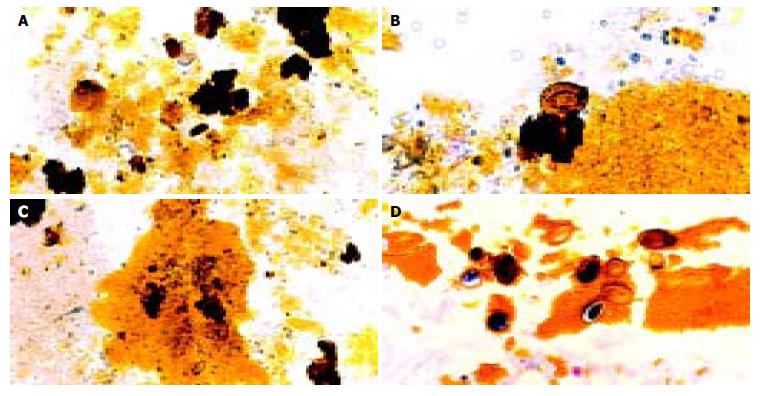
Bile examination was positive for O.viverrini eggs in 57 of the 113 cases (49.6%) studied. The presence of parasite eggs was not associated with the frequency of stones or disease association (χ2-test, P≥ 0.05). Most of the eggs were embedded in mucous gel. Aggregates of calcium bilirubinate precipitates were observed in all cases with sludge. Three cases had clear deposits of calcium bilirubinate on the eggshell, seen by special staining. Specifically, calcium was stained immediately adjacent to the eggshell, while bilirubin was observed in the outer layer (Figure 1 A, B). Examples of parasite egg-associated microcalculi aggregation and stone development are shown in Figures 2A-D.
Figure 1 Histochemical staining of biliary sludge for bilirubin (A) and calcium (B).
Calcium appears as dark deposits on the Opisthorchis eggshell (arrow) and bilirubin precipitates are demonstrated in the outer layer (arrowhead). Normal parasite eggs without deposition are shown in the same field. (A = Fouchet stain, B = von Kossa stain).
Figure 2 Typical pictures showing the cascades of Opisthorchis egg-associated stone formation starting from aggregation of the eggs admixed with mucin (A), deposition of calcium bilirubinate on the eggshells (B), and formation of tiny stones (C & D).
Original magnification, × 100 (A & C) and × 200 (B & D).
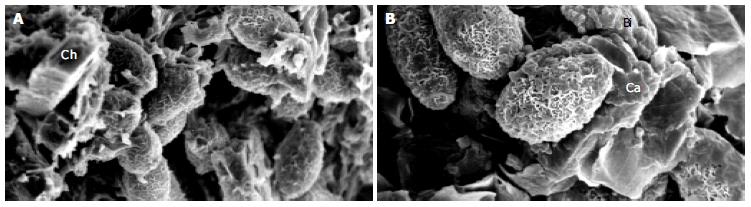
A SEM study demonstrated the presence of parasite eggs in the center of stones from all of the Opisthorchis egg-positive cases. Interestingly, 3 of the 5 egg-negative cases contained the parasite eggs in the stones. Numerous crystals, morphologically consistent with calcium derivatives and cholesterol precipitates, were observed. Typical SEM pictures of the stones are shown in Figures 3A, B.
Figure 3 SEM micrographs of gallstones showing Opisthorchis eggs with typical musk-melon-eggshell surface in the nidi of the stones.
Several crystalline structures consistent with calcium (Ca), bilirubin derivatives (Bi) and cholesterol (Ch) could be noted (A, B). Higher magnification with highlighting calcium bilirubinate deposition on Opisthorchis eggshell and mucus is shown in Figure 3B.
DISCUSSION
Biliary lithiasis is a common indication for cholecystectomy in most parts of the world. However, the prevalence and types of stones differ geographically[16-18]. Pigment stones, mostly containing calcium bilirubinate, generally constitute 10 to 27 percent of all gallstones in America and Europe, the predominant type was cholesterol stones[19-21]. In contrast, the prevalence of pigment stones is high in Asia, accounting for ≤ 90% of all stones in some parts of China[22]. In our study, almost all the cases (98.8%) of biliary lithiasis involved pigment stones. This is probably the highest reported relative frequency of pigment stones found during cholecystectomy. It exceeds a report from Bangkok, Central Thailand, where pure cholesterol stones represent ≤ 32% of stones[23]. Stones in our study of Northeast Thailand, however, occurred more frequently in females than in males as in other countries[19-20].
The association between certain parasitic infections and biliary stone formation is well documented[7,24-27]. Our study demonstrated Opisthorchis eggs in the sludge from the gallbladder bile confirmed the sludge seen in other ultrasound studies performed in Northeast Thailand[12-14]. However, our study failed to demonstrate the association between O.viverrini infection and the presence of stones, perhaps because of previous self-treatment with praziquatel, an anthelminthic widely used in Northeast Thailand[2]. We collected the treatment data, but they were not reliable as patients did not record when or how many times they took the medication before cholecystectomy. Moreover, re-infection is common in people in endemic areas.
SEM data from egg-negative patients revealed parasite eggs in the nidi of the stones, indicating these patients were previously infected. Budget constraints meant that only a limited number of cases were confirmed by SEM. More stones should be studied to test the validity of the findings.
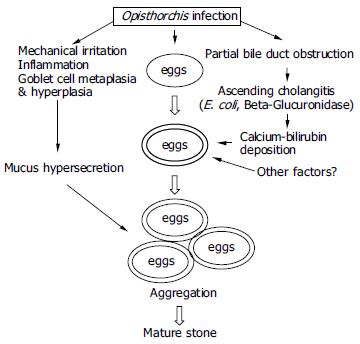
The mechanism by which parasite infection enhances pigment stone formation is not clearly understood. The present study demonstrated O.viverrini eggs in the biliary sludge and gallstones in both bile smears and the SEM, while Riganti et al[6] observed both adult worms and eggs in the nidi of gallstones from two patients. Riganti et al[7,8] also demonstrated calcium bilirubinate in pigment stones, as previously reported in stones associated with C. sinensis and ascariasis[8,27,28]. These observations have led investigators to conclude that parasite eggs and/or worms may directly stimulate stone formation[6,8]. Our histochemical findings on the calcium and bilirubin coatings on the O.viverrini eggs support this hypothesis. The presence of calcium coating on the outer surface of the parasite eggshell suggests that the eggs may act as a nucleus for stone formation. This may be similar to peripheral calcification of existing cholesterol stones[29]. Since calcium is an active element, and can precipitate several bile constituents including bilirubin, carbonate and phosphate - major components of pigment stones[30]. The parasite eggs precipitated with calcium bilirubinate and admixed with mucin, which is abundant in a liver fluke infection[3,25]. Mucin secreted by biliary epithelial cells has been recognized as an important local factor in gallstone pathogenesis[31]. This orchestrated process can eventually produce mature pigment stones as generally described[32].
Additionally, liver flukes that inhabit the bile ducts can partially obstruct the lumen leading to bile stasis and ascending cholangitis[3]. E. coli, a common bacterial species infecting the biliary system[23,33], releases β-glucuronidases, which can hydrolyze the glucuronic acid from the conjugated bilirubin[34-36]. The resulting unconjugated bilirubin precipitates as calcium salts, which is the first step in pigment stone formation[32,34]. Bile stasis is not only a condition for ascending infection but also induces stagnation of the bile components leading to stone formation[37]. Heavy Opisthorchis infection can induce poor emptying of the gallbladder[9-11]. In addition, our recent report has shown that severe fibrosis of the gallbladder wall is the main histopathology of chronic opisthorchiasis[38]. These altogether support the role of this liver fluke in gallbladder stasis, ascending cholangitis and enhanced stone formation. From previous data and our own observations, a proposed pathogenesis of Opisthorchis-associated pigment stone formation is presented in Figure 4.
Figure 4 Diagram showing the proposed pathogenesis of Opisthorchis-associated biliary stone.
In conclusion, Northeast Thailand has a high incidence of pigment stones compared to cholesterol stones at cholecystectomy. Our study clearly demonstrates that calcium bilirubinate precipitates on parasite eggshells both in light and SEM studies. This supports the role of O.viverrini infection in the pathogenesis of pigment stone formation.