Published online Nov 15, 2004. doi: 10.3748/wjg.v10.i22.3303
Revised: March 4, 2004
Accepted: March 18, 2004
Published online: November 15, 2004
AIM: To investigate the effect of actin microfilament on potassium current and hyposmotic membrane stretch-induced increase of potassium current in gastric antral circular myocytes of guinea pig.
METHODS: Whole-cell patch clamp technique was used to record potassium current in isolated gastric myocyes.
RESULTS: When the membrane potential was clamped at -60 mV, an actin microfilament disruptor, cytochanlasin-B(Cyt-B, 20 μmol/L in pipette) increased calcium-activated potassium current (IK(Ca)) and delayed rectifier potassium current (IK(V)) to 138.4% ± 14.3% and 142.1% ± 13.1% respectively at +60 mV. In the same condition, an actin microfilament stabilizer phalloidin(20 μmol/L in pipette) inhibited IK(Ca) and IK(V) to 74.2% ± 7.1% and 75.4% ± 9.9% respectively. At the holding potential of -60 mV, hyposmotic membrane stretch increased IK(Ca) and IK(V) by 50.6% ± 9.7% and 24.9% ± 3.3% at +60 mV respectively. In the presence of cytochalasin-B and phalloidin (20 μmol/L, in the pipette) condition, hyposmotic membrane stretch also increased IK(Ca) by 44.5% ± 7.9% and 55.7% ± 9.8% at +60 mV respectively. In the same condition, cytochalasin-B and phalloidin also increased IK(V) by 23.0% ± 5.5% and 30.3% ± 4.5% respectively. However, Cyt-B and phalloidin did not affect the amplitude of hyposmotic membrane stretch-induced increase of IK(Ca) and IK(V).
CONCLUSION: Actin microfilaments regulate the activities of potassium channels, but they are not involved in the process of hyposmotic membrane stretch-induced increase of potassium currents in gastric antral circular myocytes of guinea pig.
- Citation: Li XL, Zheng HF, Jin ZY, Yang M, Li ZL, Xu WX. Effect of actin microfilament on potassium current in guinea pig gastric myocytes. World J Gastroenterol 2004; 10(22): 3303-3307
- URL: https://www.wjgnet.com/1007-9327/full/v10/i22/3303.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i22.3303
Cytoskeleton is an intracellular superstructure that consists of microfilaments of actin and associated proteins, microtubules, and intermediate filaments. Actin microfilaments of the cytoskeleton form a complex network, providing the structural basis for simultaneous interactions between multiple cellular structures. It is well established that many ion channels and transporters are anchored in the membrane by either direct or indirect association with the cytoskeleton. In addition, there is growing evidence that altering the integrity of cytoskeletal elements, in particular actin microfilaments, could modulate the activity of a variety of ion channels[1] and receptors[2]. Many previous studies have demonstrated that actin microfilament could mediate different types of potassium channels of a variety of cells such as those in rat collecting duct[3], smooth muscle cell line DDT1 MF-2[4], cardiac myocytes of guinea pig[5], human meningioma cells[6], rat hippocampal CA1 pyramidal neurons[7], rat ventricular myocytes[8] and xenopus oocytes[9]. Wang et al[10] reported that neither the microfilaments nor the microtubules were involved in the enhancement of IK(V) induced by cell distension in ventricular muscle cells of guinea pig. However, Ribeiro et al[11] showed that microtubule was involved in the cell volume-induced changes in K+ transport across the rat colon epithelial cells. Our previous study demonstrated that main outward current was carried by calcium-activated potassium channel and delayed rectifier potassium channel in gastric antral circular myocytes of guinea pig and hyposmotic cell swelling enhanced the activity of that two kinds of potassium channel[12,13]. In order to investigate the mechanism of hyposmotic membrane stretch-induced increase of potassium current, in the present study, the effect of actin microfilament on potassium currents was observed and the possibility whether acin microfilament was involved in the process of hyposmotic membrane stretch-induced increase of potassium currents was examined.
Fresh, single smooth muscle cells (SMCs) were isolated enzymatically from the circular layer of guinea pig antrum as previously described[14]. Isolated SMCs were stored at 4 °C KBS until the time of use. All experiments were performed within 12 h after cell dispersion. The isolated cells were transferred to a small chamber (0.1 mL) on the stage of an inverted microscope (IX-70 Olympus, Japan) for 10-15 min to settle down. Solution was perfused at a speed of 0.9-1.0 mL/min through the chamber by gravity from the 8-channel perfusion system (L/M-sps-8; list electronics, Germany). Glass pipette with a resistance of 3-5 MΩ was used to make a giga seal of 5-10 GΩ the whole-cell currents were recorded with an Axopatch 1-D patch-clamp amplifier (Axon Instrument, USA).
Tyrode solution containing (mmol /L) NaCl 147, KCl 4, MgCl2.6H2O 1.05, CaCl2. 2H2O 0.42, Na2PO4. 2H2O 1.81, and 5.5 mmol/L glucose was used. Ca2+-free PSS containing (mmol/L) NaCl 134.8, KCl 4.5, glucose 5, and N-[2-hydroxyethyl] piperazine- N-[2-ethanesulphonic acid] (HEPES) 10 was adjusted to pH7.4 with Tris [hydroxymethyl] aminomethane (TRIZMA). Modified K-B solution containing (mmol/L) L-glutamate 50, KCl 50, taurine 20, KH2PO4 20, MgCl2 . 6H2O 3, glucose 10, HEPES 10 and egtazic acid 0.5 was adjusted to pH7.40 with KOH. Isosmotic solution (290 mOsmol/L) containing (mmol/L) NaCl 80, KCl 4.5, HEPES 10, MgCl2.6H2O 1, CaCl2.2H2O 2, Glucose 5, Sucrose 110, was adjusted to pH7.4 with Tris. Hypoosmotic solution (200 mOsmol/L) contained (in mmol/L) sucrose 30, and other ingredients was the same as the isosmotic solution. Pipette solution recording IK(Ca) contained (mmol/L) potassium-aspartic acid 110, Mg-ATP 5, HEPES 5, MgCl2.6H2O 1.0, KCl 20, egtazic acid 0.1, di-tris-creatine phoshate 2.5, disodium-creatine phosphate 2.5 and its pHwas adjusted to 7.3 with KOH. Pipette solution recording IK(V) contained (mmol/L) EGTA 10, and other ingredients was the same as the pipette solution recording IK(Ca). Cytochalasin-B was dissolved in dimethyl sulphoxide (DMSO, 20 mmol/L) and phalloidin was dissolved in alcohol (1 mmol/L). The same amount of DMSO or alcohol as the final experimental solution was added to the pipette solution. All the chemicals in this experiment were purchased from Sigma (USA).
All data were expressed as mean ± SD. Statistical significance was evaluated by a t-test. Differences were considered to be significant when P value was less than 0.05.
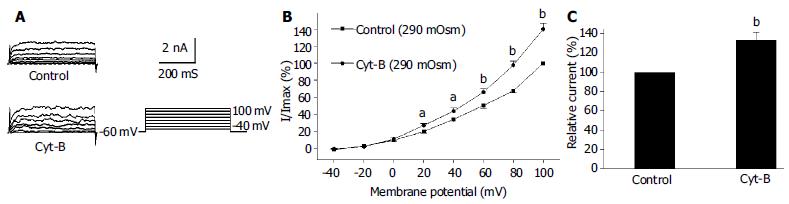
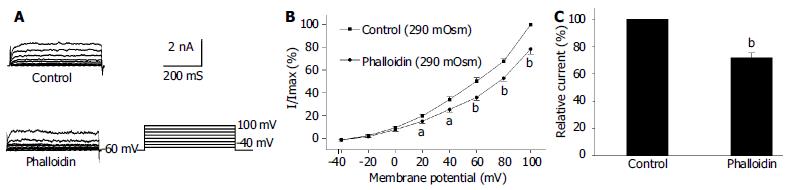
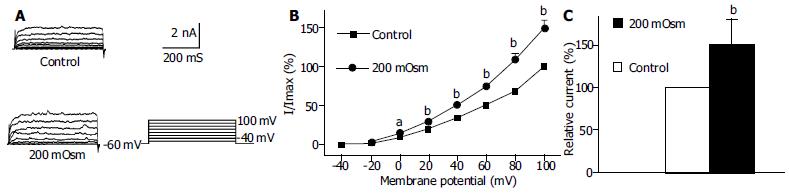
Under the whole cell configuration, the membrane potential was clamped at -60 mV, IK(Ca) was elicited by step voltage command pulse from -40 mV to +100 mV for 440 ms with a 20 mV increment at 10 s intervals. An actin microfilament disruptor, Cyt-B (20 μmol/L in pipette) markedly increased IK(Ca) to 138.4% ± 14.3% at +60 mV (n = 15, Figures 1B, C). In the same condition, an actin microfilament stabilizer, phalloidin (20 μmol/L in pipette) inhibited IK(Ca) to 74.2% ± 7.1% at +60 mV (n = 15, Figures 2B, C).
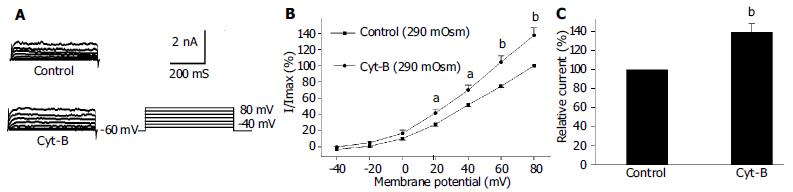
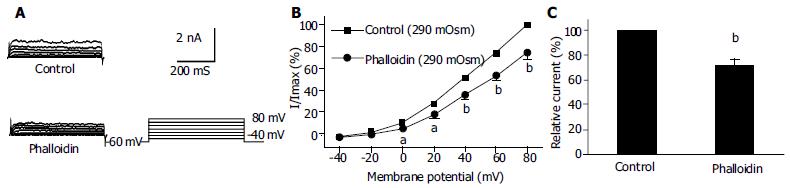
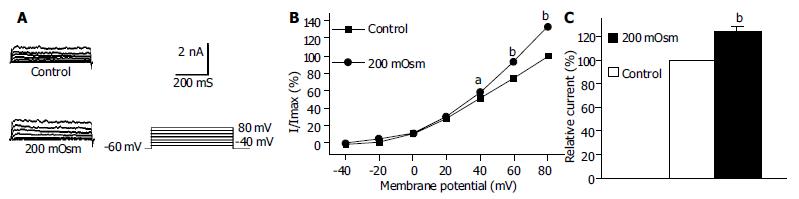
Under the whole cell configuration, the membrane potential was clamped at -60 mV, IK(V) was elicited by step voltage command pulse from -40 mV to +80 mV for 440 ms with a 20 mV increment at 10 s intervals. Cyt-B (20 μmol/L in pipette) markedly increased IK(V) to 142.1% ± 13.1% at +60 mV (n = 12, Figures 3B, C). In the same condition, phalloidin (20 μmol/L in pipette) inhibited IK(V) to 75.4% ± 9.9% at +60 mV (n = 12, Figures 4B, C).
Using the same pulse protocol, the effect of hyposmotic membrane stretch on IK(Ca) and IK(V) was observed. When the cells were superfused with hyposmotic solution (200 mOsmol/L), step command pulse-induced IK(Ca) increased from 0mV (Figure 5B)and the increasing amplitude was 50.6% ± 9.7% at +60 mV (n = 15, Figure 5C). In the same condition, hyposmotic superfusing increased step command pulse-induced IK(V) from +40 mV (Figure 6B) and the increasing amplitude was 24.9% ± 3.3% at +60 mV (n = 12, Figure 6C).
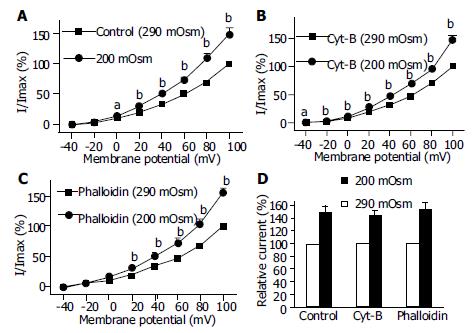
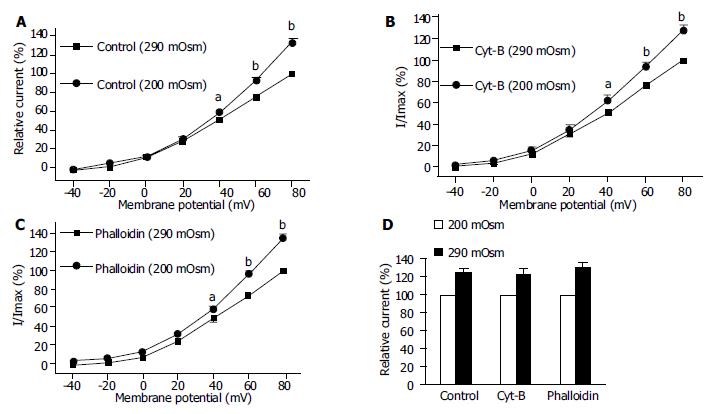
To determine the possibility of actin microfilament involved in hyposmotic membrane stretch-induced increase of IK(Ca), the effects of Cyt-B and phalloidin on IK(Ca) in which cells were perfused with isosmotic and hypoosmotic solutions were observed respectively. Hyposmotic membrane stretch increased IK(Ca) from 0 mV (Figure 7A) and the increasing amplitude was 50.6% ± 9.7% at 60 mV in the control group (n = 15, Figures 7A, Figures 8D). In the presence of Cyt-B and phalloidin (20 μmol/L in pipette) hyposmotic membrane stretch also increased IK(Ca) by 44.5% ± 7.9% (n = 15, Figures 7B, D) and 55.7% ± 9.8% (n = 15, Figures 7C, D) at +60 mV respectively. There was no significant difference between control group and Cyt-B group or phalloidin group.
Hyposmotic membrane stretch increased IK(V) by 24.9% ± 3.3% at +60 mV in the control group (n = 12, Figures 8A, D). In the presence of Cyt-B and phalloidin (20 μmol/L in pipette) hyposmotic membrane stretch also increased IK(V) by 22.9% ± 5.5% (n = 12, Figures 8B, D) and 30.3% ± 4.5% (n = 12, Figures 8C, D) at +60 mV respectively. There was no significant difference between control group and Cyt-B group or phalloidin group.
Cytoskeleton is an intracellular superstructure that consists of microfilaments of actin and associated proteins, microtubules, and intermediate filaments. Actin microfilament, in particular, are involved in structural support and a functional role in cell motility[15]. Recent evidence indicated, however, actin-based cytosleleton was involved in the control of ion channel activity across the plasma membranes of different cell types. For example, actin microfilaments were implicated in the regulation of soldium channels in human jejunal circular smooth muscle cells[16] and ATP-sensitive potassium channel in ventricular myocytes[5,17]. Actin microfilaments could also regulate voltage-dependent channels, for example, actin microfilaments could mediate voltage-dependent epithelial soldium channels in neuron cells[18].
It was proposed that cell surface proteins and extra cellular matrix were linked to the cytoskeleton by transmembrane proteins and modulate ion channels and enzymes by mechanical deformation under physiological conditions. In the present study, we observed that an actin microfilament disruptor, Cyt-B increased IK(Ca) and IK(V) significantly (Figures 1B, C, Figures 3B, C). However, an actin microfilament stabilizer, phalloidin inhibited IK(Ca) and IK(V) markedly (Figures 2B, C, Figures 4B, C) in gastric myocytes. These results suggested that when actin microfilaments were disrupted, IK(Ca) or IK(V) could be activated; while, when actin microfilaments were stabilized, IK(Ca) or IK(V) could be inhibited in gastric myocytes. Many previous studies also supported our experiment. For example, Cyt-D activated calcium-activated potassium channel in human meningioma cells[6], Cyt-B activated K (ATP) channels in cardiac[5].
Stretch is a physiological stimulation in gut smooth mucles. There are two kinds of potassium current, calcium-activated potassium current and delayed rectifier potassium current. In the present study, the two kinds of potassium current were activated by hyposmotic swelling in gastric antral smooth muscle cells of guinea pigs (Figures 5, Figures 6). In order to investigate the mechanism of hyposmotic membrane stretch-induced increase of IK(Ca) and IK(V), the relationship between potassium channel activity and actin microfilaments was observed. When actin microfilaments were disrupted by Cyt-B or stabilized by phalloidin, hyposmotic membrane stretch-induced increase of IK(Ca) and IK(V) was not affected (Figures 7, Figures 8). These results indicated that actin microfilaments were not involved in the increase of potassium current induced by hyposmotic cell swelling in gastric circular myocytes of guinea pig. Previous studies supported our results. For example, Wang et al[10] observed that neither the microfilaments nor the microtubules were involved in the enhancement of IK(V) induced by cell distension in ventricular myocytes of guinea pig. We also observed that unsaturated fatty acids, exogenous and endogenous, were involved in the increase of calcium-activated potassium current induced by hyposmotic membrane stretch(data not shown). So that hyposmotic membrane stretch-induced increase of potassium currents may be related to unsaturated fatty acids in cell membranes.
Our previous study demonstrated that actin microfilaments played an important role in the modulation of membrane stretch-induced calcium influx and hyposmotic membrane stretch-induced increase of muscarinic current in guinea-pig gastric myocytes[19,20]. It is obvious that cytoskeleton plays a different role in different types of cells and different kinds of ion channels. In gastric smooth muscle actin microfilaments may be involved in the process of hyposmotic membrane stretch-induced depolarization of membrane potential. However, actin microfilaments would not be involved in the process of cell swelling-induced hyperpolarization of membrane potential.
In summary, actin microfilaments regulate potassium channel activities in normal condition. However, actin microfilaments are not involved in hyposmotic cell swelling-induced increase of potassium currents.
Edited by Wang XL Proofread by Xu FM
| 1. | Janmey PA. The cytoskeleton and cell signaling: component localization and mechanical coupling. Physiol Rev. 1998;78:763-781. [PubMed] |
| 2. | Bowles NE, Richardson PJ, Olsen EG, Archard LC. Detection of Coxsackie-B-virus-specific RNA sequences in myocardial biopsy samples from patients with myocarditis and dilated cardiomyopathy. Lancet. 1986;1:1120-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 362] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 3. | Wang WH, Cassola A, Giebisch G. Involvement of actin cytoskeleton in modulation of apical K channel activity in rat collecting duct. Am J Physiol. 1994;267:F592-F598. [PubMed] |
| 4. | Ehrhardt AG, Frankish N, Isenberg G. A large-conductance K+ channel that is inhibited by the cytoskeleton in the smooth muscle cell line DDT1 MF-2. J Physiol. 1996;496:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Jovanović S, Jovanović A. Diadenosine tetraphosphate-gating of cardiac K(ATP) channels requires intact actin cytoskeleton. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:276-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Kraft R, Benndorf K, Patt S. Large conductance Ca(2+)-activated K(+) channels in human meningioma cells. J Membr Biol. 2000;175:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Huang H, Rao Y, Sun P, Gong LW. Involvement of actin cytoskeleton in modulation of Ca(2+)-activated K(+) channels from rat hippocampal CA1 pyramidal neurons. Neurosci Lett. 2002;332:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Shimoni Y, Ewart HS, Severson D. Insulin stimulation of rat ventricular K+ currents depends on the integrity of the cytoskeleton. J Physiol. 1999;514:735-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Song DK, Ashcroft FM. ATP modulation of ATP-sensitive potassium channel ATP sensitivity varies with the type of SUR subunit. J Biol Chem. 2001;276:7143-7149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Wang Z, Mitsuiye T, Noma A. Cell distension-induced increase of the delayed rectifier K+ current in guinea pig ventricular myocytes. Circ Res. 1996;78:466-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Ribeiro R, Heinke B, Diener M. Cell volume-induced changes in K+ transport across the rat colon. Acta Physiol Scand. 2001;171:445-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Li Y, Xu WX, Li ZL. Effects of nitroprusside, 3-morpholino-sydnonimine, and spermine on calcium-sensitive potassium currents in gastric antral circular myocytes of guinea pig. Acta Pharmacol Sin. 2000;21:571-576. [PubMed] |
| 13. | Piao L, Li Y, Li L, Xu WX. Increment of calcium-activated and delayed rectifier potassium current by hyposmotic swelling in gastric antral circular myocytes of guinea pig. Acta Pharmacol Sin. 2001;22:566-572. [PubMed] |
| 14. | Yu YC, Guo HS, Li Y, Piao L, Li L, Li ZL, Xu WX. Role of calcium mobilization in sodium nitroprusside-induced increase of calcium-activated potassium currents in gastric antral circular myocytes of guinea pig. Acta Pharmacol Sin. 2003;24:819-825. [PubMed] |
| 15. | Stossel TP. On the crawling of animal cells. Science. 1993;260:1086-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 742] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 16. | Strege PR, Holm AN, Rich A, Miller SM, Ou Y, Sarr MG, Farrugia G. Cytoskeletal modulation of sodium current in human jejunal circular smooth muscle cells. Am J Physiol Cell Physiol. 2003;284:C60-C66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Terzic A, Kurachi Y. Actin microfilament disrupters enhance K(ATP) channel opening in patches from guinea-pig cardiomyocytes. J Physiol. 1996;492:395-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Srinivasan Y, Elmer L, Davis J, Bennett V, Angelides K. Ankyrin and spectrin associate with voltage-dependent sodium channels in brain. Nature. 1988;333:177-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 312] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 19. | Xu WX, Kim SJ, So I, Kim KW. Role of actin microfilament in osmotic stretch-induced increase of voltage-operated calcium channel current in guinea-pig gastric myocytes. Pflugers Arch. 1997;434:502-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Wang ZY, Yu YC, Cui YF, Li L, Guo HS, Li ZL, Xu WX. Role of actin microfilament in hyposmotic membrane stretch-induced increase in muscarinic current of guinea-pig gastric myocytes. Shengli Xuebao. 2003;55:177-182. [PubMed] |