Published online Nov 1, 2004. doi: 10.3748/wjg.v10.i21.3191
Revised: December 22, 2003
Accepted: December 29, 2003
Published online: November 1, 2004
AIM: To investigate the photocatalytic killing effect of photoexcited TiO2 nanoparticles on human colon carcinoma cell line (Ls-174-t) and to study the mechanism underlying the action of photoexcited TiO2 nanoparticles on malignant cells.
METHODS: Ls-174-t human colon carcinoma cells were cultured in RPMI 1640 medium supplemented with 199 mL/L calf serum in a humidified incubator with an atmosphere of 50 mL/L CO2 at 37 °C. Viable cells in the samples were measured by using the MTT method. A GGZ-300 W high pressure Hg lamp with a maximum ultraviolet-A (UVA, 320-400 nm) irradiation peak at 365 nm was used as light source in the photocatalytic killing test.
RESULTS: The photocatalytic killing of Ls-174-t cells was carried out in vitro with TiO2 nanoparticles. The killing effect was weak by using UVA irradiation without TiO2 nanoparticles. In our studies, the photocatalytic killing effect was correlated with the concentration of TiO2 and illumination time. Once TiO2 was added, Ls-174-t cells were killed at a much higher rate. In the presence of 1 000 μg/mL TiO2, 44% of cells were killed after 10 min of UVA irradiation, and 88% of cells were killed after 30 min of UVA irradiation.
CONCLUSION: When the concentration of TiO2 is below 200 μg/mL, the photocatalytic killing effect on human colon carcinoma cells is almost the same as that of UVA irradiation alone. When the concentration of TiO2 is above 200 μg/mL, the remarkable killing effect of photoexcited TiO2 nanoparticles can be found.
-
Citation: Zhang AP, Sun YP. Photocatalytic killing effect of TiO
2 nanoparticles on Ls-174-t human colon carcinoma cells. World J Gastroenterol 2004; 10(21): 3191-3193 - URL: https://www.wjgnet.com/1007-9327/full/v10/i21/3191.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i21.3191
The application of TiO2 photocatalysis has received increasing attention since the first report of microbiocidal effects by Matsunaga et al[1] in 1985. In recent years, in contrast to many studies using TiO2 powder for photodecomposition of organic pollutants[2-9], few studies have investigated the application of TiO2 in life science, especially in the field of cancer treatment[10-12]. The incidence of colon cancer is rising in China. Despite that surgical operation is used currently, people have recognized its limitation. The way to treat cancer usually includes radiation therapy and chemical therapy, which may generate severe side effects in human body. Therefore, this study tried to investigate a new therapy for cancer. Ls-174-t cells were used as experiment objects in this study. The photocatalytic killing effect of TiO2 nanoparticles on malignant cells and its killing mechanism were investigated.
TiO2 colloid solutions were prepared[13,14] by hydrolysis of titanium isopropoxide, Ti [OCH (CH3) 2]4 (97%, Aldrich Chemical Co). In brief, 12.5 mL of Ti [OCH (CH3) 2]4 was added to 2 mL isopropanol, then the mixture was added to 150 mL of distilled deionized water containing 2 mL of 700 mL/L nitric acid and vigorously stirred for 6 h at 75 °C. Approximately 150 mL of TiO2 colloid solution being stable for several months at 4 °C was obtained after the organic layer was removed. The average diameter determined by Zetasizer 3000HSA (USA) was 21.2 nm.
The pH value of TiO2 colloid solutions used in the subsequent experiment had to be adjusted from 1.8 to 5.5-6.5 in order not to damage the normal growth of cells. Therefore, 1 mol/L NaOH aqueous solution and 1.5 mL/L polyvinyl alcohol were added to the colloid solutions before the pH adjustment to prevent the TiO2 from precipitation. The final TiO2 colloid solutions were sterilized by autoclaving and then diluted to the required concentration. Other chemical reagents used were all of analytical purity from commercial sources.
Human colon carcinoma cell lines Ls-174-t were purchased from Shanghai Institute of Cell Biology, Chinese Academy of Sciences, Shanghai, China. The cell lines were cultured in RPMI 1640 (Gibco) medium supplemented with calf serum 100 ml/L, penicillin (100 × 10 3μ/L) and streptomycin (100 mg/L). pH was maintained at 7.2-7.4 by equilibration with 50 mL/L CO2 . Temperature was maintained at 37 °C. Cells were sub-cultured with a mixture of ethylenedinitrile tetraacetic acid (EDTA) and trypsin. All experiments were performed using cells during the exponential growth phase. Cell concentration was determined by using a hemocytometer and the cell density was adjusted to the required final concentration.
Ls-174-t cells were treated with TiO2 diluted in RPMI 1640 medium for 2 h at 37 °C. Then the solutions were irradiated with a GGZ-300W high pressure Hg lamp (Emax = 365 nm) at room temperature. A UV pass filter was used to obtain a light wavelength between 300-400 nm. The light intensity at the liquid surface was measured by a VLX-3W radiometer-photometer (USA). The incident light intensity was 3.7 mW/cm2. In our study, three groups of tests were carried out. One group was treated in the absence of TiO2. Another group was treated in the absence of UVA. The third group was treated with different TiO2 concentrations and irradiated by UVA.
Viable cells in the samples were measured by using the MTT staining method[15]. MTT [3- (4, 5-dimethylthiazol-2-yl) -2, 5-diphenyl tetrazolium bromide] was dissolved in phosphate-buffered saline ( PBS, pH7.4) at 5 mg/mL and filtered to be sterilized. Twenty microliters of stock MTT solution were added to all wells for an assay, and plates were incubated at 37 °C for 4 h. One hundred and fifty microliters of DMSO were added to all wells and mixed thoroughly to dissolve the blue-violet crystals. After a few minutes at room temperature to ensure that all crystals were dissolved, the plates were read on a Bio-Rad Novapathtm microplate reader (Japan), using a test wavelength of 595 nm, taking the solution without MTT as control. Then optical absorptions [A] were obtained. Plates were normally read within 1 h after DMSO was added. The survival rate could be calculated according to [A]t/[A]i, where [A]i is the optical absorption of untreated cells.
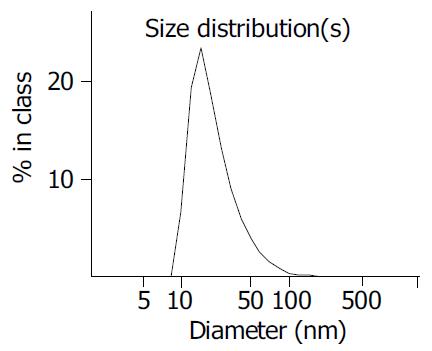
Zetasizer 3000HSA (USA) was used to determine the average diameter of TiO2 nanoparticles. The result is shown in Figure 1. The average volume size of TiO2 nanoparticles was 21.2 nm.
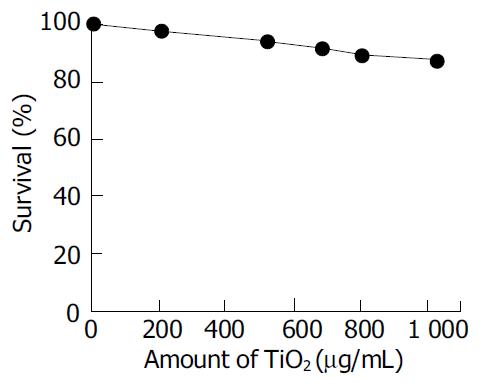
The cytotoxicity of TiO2 nanoparticles (without UVA irradiation) was determined by exposing cells to various concentrations of TiO2 in RPMI 1640 (Gibco) medium for 24 h. The surviving fraction of the cells was greater than 90% when the concentration of TiO2 was in the range of 1 000 μg/mL, as shown in Figure 2. The result confirmed that nonirradiated TiO2 nanoparticles were not toxic to the Ls-174-t cells. It was consistent with those in literatures[16-17].
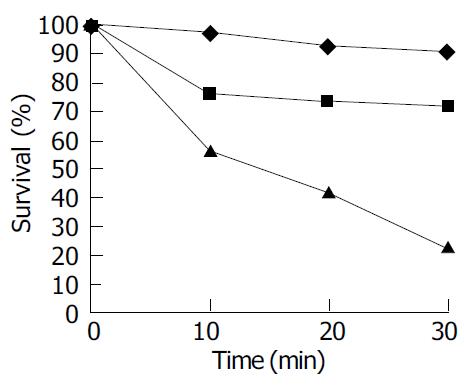
The fact that the surviving fraction was greater than 90% after 30 min as shown in Figure 3 (A) indicated that TiO2 nanoparticles without UVA irradiation showed little toxicity to living cells. The killing effect of UVA without TiO2 is shown in (B) with the surviving fraction of Ls-174-t cells given as a function of the UVA light irradiation time. About 20% cells were killed after 30 min exposure, whereas after 20 min exposure, more than 90% of the cells survived. Once TiO2 was added, the Ls-174-t cells were killed at a much higher rate as shown in (C). For example, in the presence of 1 000 μg/mL of TiO2, 44% of the cells were killed after 10 min of UVA irradiation, and after 30 min irradiation, 80 % of the cells were killed. Therefore it was concluded that photoexcited TiO2 nanoparticles had an active killing effect on Ls-174-cells.
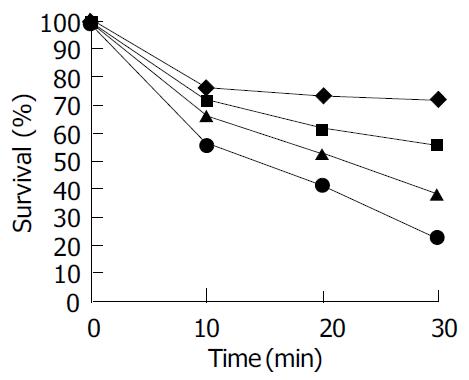
The effect of the TiO2 concentration ranging from 200 to 1 000 μg/mL on the rate of cell killing by UVA light is shown in Figure 4. The light intensity was 3.7 mW/cm2 and kept constant. The experimental results demonstrated that cell viability decreased monotonically as TiO2 concentration increased and cell viability decreased with time.
Although a higher concentration of TiO2 could achieve a higher reaction rate, the difficulties in separation and measurement had to be considered. So the effect of a higher TiO2 concentration (C > 1 029 μg/mL) on Ls-174-t cell activity was not investigated. The concentration of TiO2 was set at < 1 029 μg/mL with which the dark cytotoxicity was considered to be negligible. The range of TiO2 concentrations was close to that used previously[18].
Untreated and TiO2-treated cells were collected by centrifugation and resuspended in RPMI 1640 medium. The samples were pipetted into a 24-well plate, which was directly observed with an inverted phase-contrast microscope. When treated by photoexcited TiO2, the cellular shape was condensed and nuclei were dispersed in fragments.
In this experiment, the TiO2 nanoparticle system was used, displaying its superiority. TiO2 nanoparticles were easy to attach to the cellular membranes and accumulate. They were also easy to enter into the cytoplasm via phagocytosis[19]. It could lead to accumulation of ROS on the surface of cell membranes and in the cytoplasm. Hence under light irradiation, TiO2 nanoparticles had more significant cell killing effect in vitro.
Human colon carcinoma cells treated with photoexcited TiO2 nanoparticles (C > 200 μg/mL) were effectively damaged, with cells contracted and a lot of cell fragments simultaneously observed by an inverted phase-contrast microscope[18].According to these characteristics, we assumed that the mechanism of photoexcited TiO2 in killing human colon carcinoma cells might be through a series of oxidized chain reactions and in inducing cell death by reactive oxygen species[20-25]. Human colon carcinoma cell damages occurred in two stages. The initial oxidative damage took place on the cell membranes, where the TiO2 photocatalytic surface had its first contact with intact cells, the membranes became somewhat permeable. At this stage the cells did not lose their viability. Photocatalytic action made the cell membranes permeable, intracellular components began to leak from the cells and free TiO2 nanoparticles might also diffuse into the damaged cells and directly attack intracellular components, eventually leading to cell death. It is different from the bactericidal effect of TiO2 photocatalytic reaction. Bacteria are simple prokaryotic cells that do not contain the nucleus characteristics of eukaryotic cells. Whereas human colon carcinoma cells are eukaryotic cells and their structure is complex. Based on their structural differences, we assumed that killing cancer cells might be more difficult than killing bacteria by the photocatalytic reaction of TiO2 nanoparticles.
In the present study, cultured human colon carcinoma cells were effectively killed by photoexcited TiO2 nanoparticles in virto. The concentration of TiO2 affected the photocatalytic killing effect. When the concentration of TiO2 was below 200 μg/mL, there was only a slight decrease in survival ratio after UVA irradiation for more than 30 min. It was almost the same as that of UVA irradiation alone. It indicated that minor cell membrane leakage might occur and the cell viability was not lost. When the concentration of TiO2 was above 200 μg/mL, the survival ratio decreased rapidly with increasing TiO2 concentration. It indicated that major rupture of cell membranes and decomposition of essential intracellular components might take place, thus accelerating cell death. It verified the mechanism of TiO2 nanoparticles in killing human colon carcinoma cells.
The photocatalytic killing effect of TiO2 nanoparticles on human colon carcinoma cells suggested the idea of cancer treatment using TiO2 nanoparticles and light irradiation. Under these conditions, it could be adapted to an anticancer modality by the local or regional treatment of the tumor with TiO2 nanoparticles, followed by light irradiation focusing on the tumor. Although UVA light (320-400 nm) cannot penetrate the human body deeply, it may be possible that the modality will be applied to several human tumors in the future.
Edited by Zhang JZ and Wang XL Proofread by Zhu LH and Xu FM
| 1. | Matsunaga T, Tomoda R, Nakajima T, Wake H. Photoelectro-chemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol Lett. 1985;29:211-214. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1069] [Cited by in RCA: 1079] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 2. | Byrne JA, Eggins BR, Brown NMD, Mckinney B, Rouse M. Immobilisation of TiO2 powder for the treatment of polluted water. Appl Catal B Environ. 1998;17:25-36. [DOI] [Full Text] |
| 3. | Wang KH, Hsieh YH, Ko RC, Chang CY. Photocatalytic degrada-tion of wastewater from manufactured fiber by titanium dioxide suspensions in aqueous solution. Environ Int. 1999;25:671-676. [DOI] [Full Text] |
| 4. | Byrne JA, Eggins BR, Byers W, Brown NMD. Photoelectrochemical cell for the combined photocatalytic oxidation of organic pol-lutants and the recovery of metals from waste waters. Appl Catal B Environ. 1999;2:L85-L89. |
| 5. | Horikoshi S, Satou Y, Hidaka H, Serpone N. Enhanced photo-current generation and photooxidation of benzene sulfonate in a continuous flow reactor using hybrid TiO2 thin films immobilized on OTE electrodes. J Photochem Photobiol A. 2001;146:109-119. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Molinari R, Grande C, Drioli E, Palmisano L, Schiavello M. Photocatalytic membrane reactors for degradation of organic pollutants in water. Catal Today. 2001;67:273-279. [RCA] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Shephard GS, Stockenström S, de Villiers D, Engelbrecht WJ, Wessels GF. Degradation of microcystin toxins in a falling film photocatalytic reactor with immobilized titanium dioxide catalyst. Water Res. 2002;36:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Muneer M, Singh HK, Bahnemann D. Semiconductor-mediated photocatalysed degradation of two selected priority organic pollutants, benzidine and 1,2-diphenylhydrazine, in aqueous suspension. Chemosphere. 2002;49:193-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Alhakimi G, Studnicki LH, Al-Ghazali M. Photocatalytic de-struction of potassium hydrogen phthalate using TiO2 and sunlight: application for the treatment of industrial wastewater. J Photochem Photobiol A. 2003;154:219-228. [RCA] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Fujishima A, Cai RX, Otsuki J, Hashimoto K, Itoh K, Yamashita T, Kubota Y. Biochemical application of photoelectrochemistry: photokilling of malignant cells with TiO2 powder. Electrochim Acta. 1993;38:153-157. [RCA] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Mills A, Hunte SL. An overview of semiconductor photocatalysis. J Photochem Photobiol A. 1997;108:1-35. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2898] [Cited by in RCA: 1519] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 12. | Fujishima A, Rao TN, Tryk DA. Titanium dioxide photocatalysis. J Photochem Photobiol C. 2000;1:1-21. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6275] [Cited by in RCA: 3006] [Article Influence: 120.2] [Reference Citation Analysis (0)] |
| 13. | Kormann C, Bahnemann DW, Hoffmann MR. Preparation and characterization of quantum-size titanium dioxide. J Phys Chem. 1988;92:5196-5201. [RCA] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 384] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 14. | O'Regan B, Moser J, Anderson M, Gratzel M. Vectorial electron injection into transparent semiconductor membranes and elec-tric field effects on the dynamics of light-induced charge separation. J Phys Chem. 1990;94:8720-8726. [RCA] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 370] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 15. | Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38285] [Cited by in RCA: 39357] [Article Influence: 937.1] [Reference Citation Analysis (0)] |
| 16. | Bernard BK, Osheroff MR, Hofmann A, Mennear JH. Toxicology and carcinogenesis studies of dietary titanium dioxide-coated mica in male and female Fischer 344 rats. J Toxicol Environ Health. 1990;29:417-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 72] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Linnainmaa K, Kivipensas P, Vainio H. Toxicity and cytogenetic studies of ultrafine titanium dioxide in cultured rat liver epithelial cells. Toxicol In Vitro. 1997;11:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Xu MH, Huang NP, Xiao ZD, Lu ZH. Photoexcited TiO2 nanoparticles through . OH- radicals induced malignant cells to necrosis. Supramole Sci. 1998;5:449-451. [RCA] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Cai RX, Hashimoto K, Itoh K, Kubota Y, Fujishima A. Photokilling of malignant cells with Ultrafine TiO2 powder. Bull Chem Soc Jpn. 1991;64:1268-1273. [RCA] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 201] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 20. | Jaeger CD, Bard AJ. Spin trapping and electron spin resonance detection of radical intermediates in the photodecomposition of water at TiO2 particulate systems. J Phys Chem. 1979;83:3146-3152. [RCA] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 308] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 21. | Rao MV, Rajeshwar K, Pal Verneker VR, DuBow J. Photosyn-thetic production of H2 and H2O2 on semiconducting oxide grains in aqueous solutions. J Phys Chem. 1980;84:1987-1991. [RCA] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Harbour JR, Hair ML. Superoxide generation in the photolysis of aqueous cadmium sulfide dispersions. Detection by spin trapping. J Phys Chem. 1977;81:1791-1793. |
| 23. | Harbour JR, Tromp J, Hair ML. Photogeneration of hydrogen peroxide in aqueous TiO2 dispersions. Can J Chem. 1985;63:204-208. [RCA] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 118] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Hong AP, Bahnemann DW, Hoffmann MR. Cobalt (II) tetrasulfop-hthalocyanine on titanium dioxide: a new efficient electron relay for the photocatalytic formation and depletion of hydrogen per-oxide in aqueous suspensions. J Phys Chem. 1987;91:2109-2117. [RCA] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Hidaka H, Horikoshi S, Serpone N, Knowland J. In vitro photo-chemical damage to DNA, RNA and their bases by an inor-ganic sunscreen agent on exposure to UVA and UVB radiation. J Photochem Photobiol A. 1997;111:205-213. [RCA] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 72] [Article Influence: 2.6] [Reference Citation Analysis (0)] |