Published online Nov 1, 2004. doi: 10.3748/wjg.v10.i21.3137
Revised: March 5, 2004
Accepted: March 12, 2004
Published online: November 1, 2004
AIM: To investigate the change of immunological characteristics of HBsAg caused by the mutation at codon 145 of HBsAg using DNA-based immunization.
METHODS: Plasmids expressing mutant and wild type envelope antigens were transfected into human hepatocellular carcinoma cellsviaelectrotransformation. The antigenicity of HBsAg was studied with EIA and immunocytochemical staining. Then plasmids were used to immunize 5 C57BL/6 mice. Sera of mice were detected for anti-HBs and anti-preS2 with ELISA.
RESULTS: The mutant HBsAg could be detected by native antibody in EIA and immunocytochemical study. But the A(450 nm) value of the mutant HBsAg in the supernatant was apparently lower than that of the wild-type. Both mutant and native HBsAg expression plasmid could stimulate a strong humoral immune response to HBsAg and preS2 antigen in mice. Protective antibodies against HBsAg elicited by the native HBsAg occurred earlier than that elicited by the mutant HBsAg about one to two weeks. The occurrence of protective antibodies against preS2 antigen was one to two weeks earlier than that of anti-HBs.
CONCLUSION: The amino acid substitution causes changes of the antigenicity and immunogenicity of HBsAg, but mutant HBsAg can still induce a protective humoral immune response in mice.
-
Citation: Ge JH, Liu HM, Sun J, Zhang LZ, He J, Li YL, Liu H, Xu Y, Yu HY, Hu YP. Antigenic and immunogenic changes due to mutation of
s gene of HBV. World J Gastroenterol 2004; 10(21): 3137-3140 - URL: https://www.wjgnet.com/1007-9327/full/v10/i21/3137.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i21.3137
Hepatitis B infection is a serious problem worldwide. HBV vaccine is effective in preventing HBV infection. Antibody responses to the common epitope of HBsAg, the “a” determinant, are considered to confer protection against HBV infection regardless of viral subtypes. HBV infection still occurs in spite of the presence of anti-HBs[1-3]. Some cases were infected with a variant of HBV. This variant has a point mutation from guanosine to adenosine at nucleotide position 587 of the s gene that results in an amino acid substitution of arginine for glycine at codon 145 of HBsAg[4,5]. This mutation would greatly destroy the antigenicity of HBsAg, which directly impacts on the diagnosis and therapy of HBV[6]. Also, this variant was found in patients who had liver transplantations for end-stage liver diseases associated with HBV infection and received human monoclonal anti-HBs antibody or HBIG in an attempt to prevent recurrent hepatitis B[2,7,8]. This study was undertaken to determine whether the change from glycine to arginine at amino acid 145 of HBsAg could cause a loss of antigenicity and immunogenicity of HBsAg, thus allowing the mutant HBV to evade the humoral immune response.
Restriction endonucleases and T4 DNA ligase were obtained from Sangon Co. (Canada). Plasmid P II containing overlength HBV genomes was endowed by Dr. Jian-Wen He. Plasmid P II had a point mutation from guanosine to adenosine at the nucleotide position 587 of s gene and resulted in an aminoacid substitution of arginine for glycine at codon 145 of HBsAg. Plasmid pCMV-S2.S was a generous gift of Dr. Heather Davis (Loeb Research Institute, Ottawa, Canada). This vector contained a cytomegalovirus promoter and respiratory syncytial virus enhancer element and encoded HBsAg and MHBs proteins. Plasmid SEAP expressing alkaline phosphatase was a generous gift of Dr. Jian-Wen He. HBsAg and HBsAb ELISA reagents were purchased from Abbott Laboratories and Sino-American Biotechnology Co., respectively. PreS2 antigen and preS2-specific antibodies were measured using ELISA kits from Hepatic Disease Institute of Beijing Medical University. The mouse monoclonal antibody against HBsAg was purchased from DAKO (USA). Sheep anti-mouse IgG-HRP was obtained from CALBIOCHEM (Germany). QIA quick gene gel kit and plasmid extraction kit were purchased from QIA gene. C57BL/6 mouse strain bought from Animal Center of Shanghai Birth Control Research Institute was kept under standard pathogen-free conditions in the animal facility and maintained on a 14:10 light-dark schedule (lights off at 10 pm, on at 8 am). Mice used were aged 6-8 wk.
Plasmid P II used as the source of mutant viral gene and plasmid pCMV-S2. S used as the source of the vector were digested with Dra III and Xho I, respectively. Then the segment of mutant s gene from plasmid P II was inserted into the vector from pCMV-S2.S by T4 DNA ligase. Eukaryotic expression plasmid pCMV-S2.S + 145R containing a point mutation from guanosine to adenosine was constructed. Plasmid pCMV-S2.S + 145R was confirmed by restriction endonuclease digestion and HBV insert was sequenced by the dideoxy method using a commercial kit. The plasmid was grown in DH5 α and extracted by QIA quick gene kit. DNA was dissolved in double distilled water, adjusted to 1.6 mg/L, and then diluted to a final concentration of 1 mg/L for in vivo studies. Concentration and purity of the DNA were confirmed by measuring the optical density at 260 nm and by agarose gel electrophoresis.
Human hepatocellular carcinoma cell lines (Hep G2) were transfected with the eukaryotic expression vectors pCMV-S2.S + 145R, pCMV-S2.S or pcDNA3.0 via electrotransformation. The change of binding power of mutant antigens to anti-HBs was studied by EIA and immunocytochemical staining. To control transfection efficiency, cells were cotransfected with an alkaline-phosphatase-containing vector SEAP. Cells were lysed by freeze-thawing three times in phosphate-buffered saline (PBS), and the supernatants were collected at various time points after transfection for viral protein studies.
Concentrations of HBsAg and preS2 envelope proteins derived from culture supernatant or cell lysates of transfected cells were measured by enzyme-linked immunosorbent assay reagents according to the manufacturer’s instructions. One hundred μL of culture supernatant or cell lysates was incubated with 100 μL of 2 × SEAP buffer at 37 °C for 10 min. Twenty μL substrate buffer was added to the assay. A(450 nm) value was determined after incubated at 37 °C for 30 min. All results were corrected according to the transfection efficiency using the SEAP assays.
Transfected cells were collected by centrifugation and spread on sterile glass slides, and then fixed with acetone at 4 °C for immunochemical staining. Slides were blocked with normal goat serum for 30 min at room temperature, then incubated either with monoclonal mouse anti-HBs at a dilution of 1:200 for detection of HBsAg or with monoclonal mouse anti-preS2 (1:500) for detection of preS2 antigen for 12 h at 4 °C. After washed with PBS, a secondary antiserum consisting of biotin-conjugated goat anti-mouse immunoglobulin G was applied at a 1:100 dilution for 30 min at 37 °C. The slides coated with antibody were washed with PBS, treated with streptavidin-horseradish peroxidase conjugates at a 1:600 dilution for 30 min at 37 °C, stained with nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl-phosphate (NBT/BCIP, MAXIN Co.), and counter-stained with Mayer’s hematoxylin before mounted.
Mice were injected on a single occasion with 100 μg of recombinant plasmid DNA pCMV-S2.S, pCMV-S2.S + 145R or pcDNA3.0 in 100 μL of 0.9 g/L NaCl distributed into the anterior tibialis muscle of C57BL/6 mice 5 d after the injection of 100 μL bupivacaine (0.2%-0.4%). Immunization with the empty plasmid pcDNA3.0 vector (mock) was used as a negative control. Mice were anesthetized with pentobarbitone prior to phlebotomy from the retro-orbital plexus 200 microliter using heparinized glass pipettes every week. Serum was isolated by centrifugation. Anti-HBs and anti-preS2 concentrations were analyzed by using ELISA kits according to the manufacturer’s instructions.
Standard anti-HBs at titres 80, 40, 20, 10 or 0 mIU/L was detected by ELISA at the time when serum samples were detected. The A(450 nm) value was 0.872, 0.442, 0.225, 0.107 and 0.046, respectively. The data were analyzed by linear regression, and the equation between the A(450 nm) value and the antibody titers was y = 94.442x-1.959. According to the manufacturer’s instructions, inhibition rate (%) = (A value of negative control - A value of sample)/(A value of negative control - A value of positive control) × 100%.
The data were analyzed by SAS software.
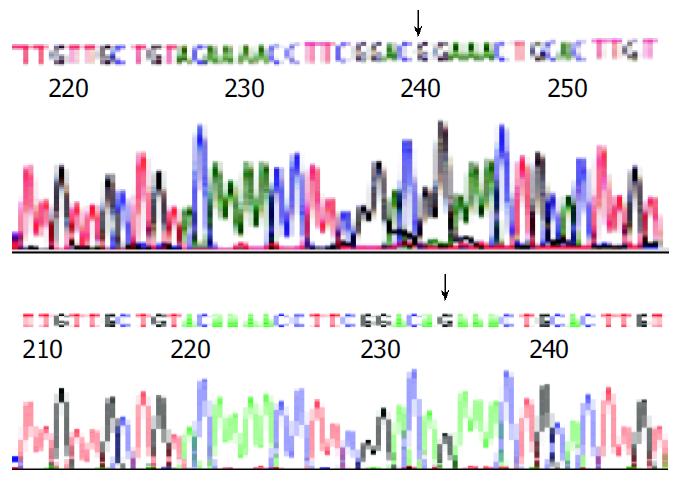
The results of endonuclease digestion and electrophoresis were in accordance to the graphic map of plasmids. The result of sequencing was same as the sequence in the other report[9], except the point mutation from guanosine to adenosine at the nucleotide position 587 of s gene (Figure 1).
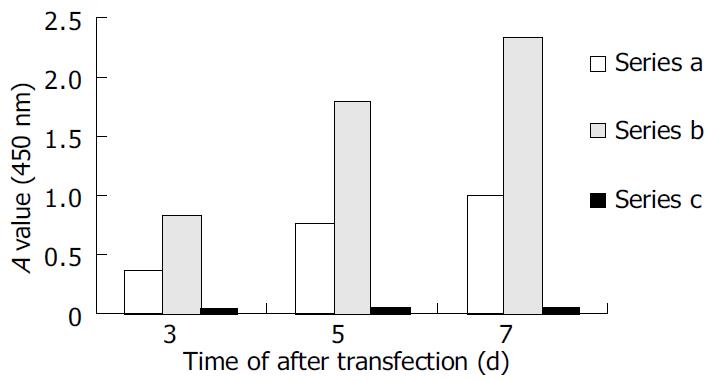
HepG2 cells were transfected with pCMV-S2.S + 145R, pCMV-S2.S or pcDNA3.0 and culture supernatant was collected at various intervals of 3, 5, 7 d after transfection. pCMV-S2.S-transfected cells secreted a higher amount of HBsAg compared with the pCMV-S2.S + 145R-transfected cells in the culture supernatant and the cell lysates of transfected cells (Figure 2). But preS2 antigen was not detected in the culture supernatant or in the cell lysates of pCMV-S2.S-transfected and pCMV-S2.S + 145R-transfected cells. In contrast, immunocytochemical studies clearly showed a substantial cytoplasmic accumulation of HBsAg and preS2 antigen in both of the pCMV-S2.S-transfected and pCMV-S2.S + 145R- transfected cells. Furthermore, there was no difference in the expression of HBsAg and preS2 antigen between the two plasmid transfected cells. No staining pattern was observed in mock-transfected cells (data not shown).
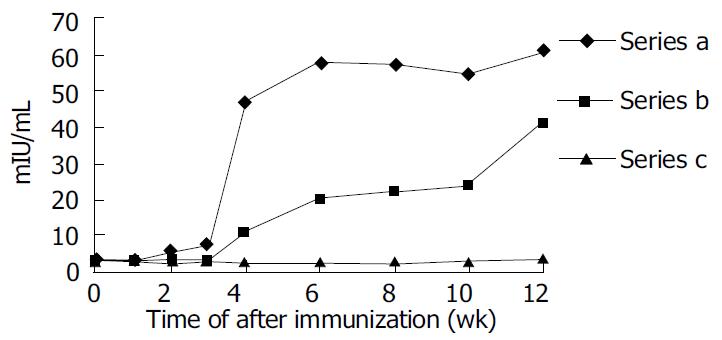
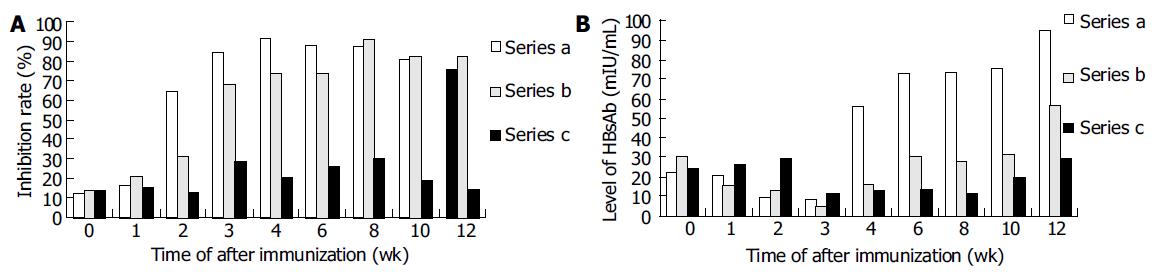
Anti-HBs responses to pCMV-S2.S + 145R were detectable 4 wk after the immunization in C57BL/6 mice. The titer of anti-HBs increased gradually by the end of observation. The highest titer was 95.60 mIU/L at the twelfth week after immunization. Most importantly, anti-HBs responses to pCMV-S2.S were detectable 2 wk after immunization in C57BL/6 mice. The titer of anti-HBs also increased gradually by the end of observation. The highest titer was 99.57 mIU/L at the twelfth week after immunization. The mice immunized with pCMV-S2.S + 145R developed anti-HBs after a delay of approximately 2 wk compared with those injected with pCMV-S2.S (Figure 3). The occurrence of protective antibodies against HBsAg elicited by native HBsAg was one to two weeks earlier than that elicited by mutant HBsAg. Nevertheless, pCMV-S2.S + 145R-immunized as well as pCMV-S2.S-immunized mice developed a strong humoral immune response against HBsAg at levels far above the known anti-HBs protection limit for humans (> 10 mU/mL). Anti-HBs response was not detected in C57BL/6 mice immunized with pcDNA3.0.
Both pCMV-S2.S + 145R-immunized and pCMV-S2.S-immunized mice developed a significant preS2-specific antibody response, whereas antibody responses to the preS2 antigen were detectable two weeks after immunization. There was a slight difference between the two groups with respect to the level of anti-preS2 antigen response (Figure 4A).
In order to analyze the difference in immune response induced by pCMV-S2.S + 145R and pCMV-S2.S and the validity of immunization and the titer of Anti-HBs were corrected according to the inhibition rate of anti-preS2 which was little affected by the point mutation, because the structures of pCMV-S2.S + 145R and pCMV-S2.S were exactly the same except for a point mutation from guanosine to adenosine at the nucleotide position 587 of s gene. Therefore, the ratio of HBsAg and preS2 antigen expressed by the two plasmids was similar. Then the difference between the two plasmids due to the mutation could be observed correctly (Figure 4B).
The antibody responses to the common “a” determinant of HBsAg confer protection against infection with HBV of all subtypes. Using monoclonal antibodies, this determinant is composed of a number of epitopes, and its tertiary structure is important for its antigenicity. The amino acid arginine, which is substituted for glycine, is a much larger residue and charged[10]. As a result the hydrophobicity profiles of this region of the two antigens are quite different and would be expected to affect the secondary and tertiary structures of the antigen. This amino acid substitution lies within the “a” determinant and so potentially alters the epitopes recognized by the protective immune response.
The secondary and tertiary structures of HBsAg expressed by the eukaryotic expression vector were most similar to those of viruses, which could exclude possible alterations of the antigenic properties by purification procedures of the recombinant vaccine. In transfection and in vitro expression study, HBsAg secreted by pCMV-S2.S-transfected cells had a higher affinity to the anti-HBs compared with that secreted by pCMV-S2.S + 145R-transfected cells in the culture supernatant and the cell lysates of transfected cells, although the transfection efficiency was corrected. The antigenicity of HBsAg was altered by this single amino acid substitution. The mutant antigen could not be totally recognized by anti-HBs induced by naive antigens. The point mutation could influence the affinity, but mutant antigen could still be detected by anti-HBs.
The immunogenicity of HBsAg was also altered by this point mutation. The anti-HBs titer to the native HBsAg induced by mutant HBsAg was significantly lower than that induced by native HBsAg, while there was no significant difference between the titers of anti-preS2 induced by the two antigens. These results support the suggestion that this mutation was the result of immune pressure[11]. This variant could evade the immune response elicited after natural infection or after vaccination[12,13].
Both HBsAg and preS2 antigens, expressed by these two plasmids, were able to induce a strong humoral immune response. Indeed, anti-HBs titers induced by both plasmids were far above 10 mIU/L. The level of anti-HBs was sufficient to protect humans against HBV infection after exposure to the virus. This DNA vaccine could also confer the protection against infection with prototype HBV.
The preS2 antigen was not detected in the culture supernatant or cell lysates of transfected cells, but a substantial cytoplasmic accumulation was detected in plasmid transfected cells by immunocytochemical study, and the anti-preS2 was also detected in mice after DNA immunization. It is likely that the preS2 antigen was a nonsecreted protein expressed by this vector in HepG2 cells. It could be a nonsecreted protein in vivo, but it was possible that bupivacaine followed by plasmid DNA injections caused early activation of nonspecific inflammatory mediators by recruiting professional APCs[14,15], B cells, and T cells. In addition, a specific immune response against muscle cells expressing viral peptides or proteins might subsequently lead to damage of muscle fibers and release of sequestered antigens. Such antigens might eventually reach lymph nodes or alternatively taken up locally by APCs. The occurrence of protective antibodies of anti-HBs2 was one to two weeks earlier than that of anti-HBs in the same group. This result was similar to other reports[16,17]. There was little difference in occurrence, high titre and duration of anti-preS2 between the mutant and native viral protein expression plasmids. It indicated that this mutation had little impact on the antigenicity and immunogenicity of preS2 antigen. Therefore, the ingredient of preS2 antigen in hepatitis B vaccine would protect humans against this mutant HBV infection after exposure to the virus, and it also could shorten the occurrence of protective antibody and give an early stage protection. This would benefit the infants born by HBeAg positive mothers and persons exposed to HBV.
In conclusion, DNA immunization with the mutant HBsAg expression vector, produces a lower affinity than that elicited by the native HBsAg expression vector. The mutation changes the antigenicity and immunogenicity of HBsAg, and also induces a protective immune response.
Co-correspondents: Le-Zhi Zhang and Yi-Ping Hu
Edited by Wang XL and Ren SY Proofread by Xu FM
| 1. | Lee KM, Kim YS, Ko YY, Yoo BM, Lee KJ, Kim JH, Hahm KB, Cho SW. Emergence of vaccine-induced escape mutant of hepatitis B virus with multiple surface gene mutations in a Korean child. J Korean Med Sci. 2001;16:359-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Kidd-Ljunggren K, Miyakawa Y, Kidd AH. Genetic variability in hepatitis B viruses. J Gen Virol. 2002;83:1267-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 216] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 3. | Nainan OV, Khristova ML, Byun K, Xia G, Taylor PE, Stevens CE, Margolis HS. Genetic variation of hepatitis B surface antigen coding region among infants with chronic hepatitis B virus infection. J Med Virol. 2002;68:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Cooreman MP, Leroux-Roels G, Paulij WP. Vaccine- and hepatitis B immune globulin-induced escape mutations of hepatitis B virus surface antigen. J Biomed Sci. 2001;8:237-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 141] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Osiowy C. Sensitive detection of HBsAg mutants by a gap ligase chain reaction assay. J Clin Microbiol. 2002;40:2566-2571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Fischer L, Kalinina T, Rogiers X, Will H, Sterneck M. GLY145ARG mutation emerging under HBIG treatment in patients with recurrent HBV after liver transplantation strongly reduces viral secretion. Transplant Proc. 2001;33:3633-3636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Rodriguez-Frias F, Buti M, Jardi R, Vargas V, Quer J, Cotrina M, Martell M, Esteban R, Guardia J. Genetic alterations in the S gene of hepatitis B virus in patients with acute hepatitis B, chronic hepatitis B and hepatitis B liver cirrhosis before and after liver transplantation. Liver. 1999;19:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Grottola A, Buttafoco P, Del Buono MG, Cremonini C, Colantoni A, Gelmini R, Morelli C, Masetti M, Jovine E, Fruet F. Pretransplantation pre-S2 and S protein heterogeneity predisposes to hepatitis B virus recurrence after liver transplantation. Liver Transpl. 2002;8:443-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Gan RB, Chu MJ, Shen LP, Qian SW, Li ZP. The complete nucleotide sequence of the cloned DNA of hepatitis B virus subtype adr in pADR-1. Sci Sin B. 1987;30:507-521. [PubMed] |
| 10. | Waters JA, Kennedy M, Voet P, Hauser P, Petre J, Carman W, Thomas HC. Loss of the common "A" determinant of hepatitis B surface antigen by a vaccine-induced escape mutant. J Clin Invest. 1992;90:2543-2547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 155] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Oon CJ, Chen WN, Goo KS, Goh KT. Intra-familial evidence of horizontal transmission of hepatitis B virus surface antigen mutant G145R. J Infect. 2000;41:260-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Hou J, Wang Z, Cheng J, Lin Y, Lau GK, Sun J, Zhou F, Waters J, Karayiannis P, Luo K. Prevalence of naturally occurring surface gene variants of hepatitis B virus in nonimmunized surface antigen-negative Chinese carriers. Hepatology. 2001;34:1027-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 155] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Chen WN, Oon CJ. Hepatitis B virus surface antigen (HBsAg) mutants in Singapore adults and vaccinated children with high anti-hepatitis B virus antibody levels but negative for HBsAg. J Clin Microbiol. 2000;38:2793-2794. [PubMed] |
| 14. | Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, Gromkowski SH, Deck RR, DeWitt CM, Friedman A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1713] [Cited by in RCA: 1616] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 15. | Oka Y, Akbar SM, Horiike N, Joko K, Onji M. Mechanism and therapeutic potential of DNA-based immunization against the envelope proteins of hepatitis B virus in normal and transgenic mice. Immunology. 2001;103:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Geissler M, Tokushige K, Chante CC, Zurawski VR, Wands JR. Cellular and humoral immune response to hepatitis B virus structural proteins in mice after DNA-based immunization. Gastroenterology. 1997;112:1307-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Madalinski K, Sylvan SP, Hellström U, Mikolajewicz J, Zembrzuska-Sadkowska E, Piontek E. Antibody responses to preS components after immunization of children with low doses of BioHepB. Vaccine. 2001;20:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |