Published online Oct 15, 2004. doi: 10.3748/wjg.v10.i20.3021
Revised: January 23, 2004
Accepted: February 24, 2004
Published online: October 15, 2004
AIM: To evaluate the therapeutic effect of adenoviral-vector-delivered human interleukin-10 (hIL-10) gene on severe acute pancreatitis (SAP) rats.
METHODS: Healthy Sprague-Dawley (SD) rats were intraperitoneally injected with adenoviral IL-10 gene (AdvhIL-10), empty vector (Adv0) or PBS solution. Blood, liver, pancreas and lung were harvested on the second day to examine hIL-10 level by ELISA and serum amylase by enzymatic assay. A SAP model was induced by retrograde injection of sodium taurocholate through pancreatic duct. SAP rats were then administered with AdvhIL-10, Adv0 and PBS solution by a single intraperitoneal injection 20 min after SAP induction. In addition to serum amylase assay, levels of hIL-10 and tumor necrosis factor-α (TNF-α ) were detected by RT-PCR, ELISA and histological study. The mortality rate was studied and analyzed by Kaplan–Meier and log rank analysis.
RESULTS: The levels of hIL-10 in the pancreas, liver and lung of healthy rats increased significantly after AdvhIL-10 injection (1.42 ng/g in liver, 0.91 ng/g in pancreas); while there was no significant change of hIL-10 in the other two control groups. The concentration of hIL-10 was increased significantly in the SAP rats after AdvhIL-10 injection (1.68 ng/g in liver, 1.12 ng/g in pancreas) compared to the other two SAP groups with blank vector or PBS treatment (P < 0.05). The serum amylase levels remained normal in the AdvhIL-10 transfected healthy rats. However, the serum amylase level was significantly elevated in the other two control SAP rats. In contrast, serum amylase was down-regulated in the AdvhIL-10 treated SAP groups. The TNF-α expression in the AdvhIL-10 treated SAP rats was significantly lower compared to the other two control SAP groups. The pathohistological changes in the AdvhIL-10 treated group were better than those in the other two control groups. Furthermore, the mortality of the AdvhIL-10 treated group was significantly reduced compared to the other two control groups (P < 0.05).
CONCLUSION: Adenoviral hIL-10 gene can significantly attenuate the severity of SAP rats, and can be used in the treatment of acute inflammation process.
- Citation: Chen ZQ, Tang YQ, Zhang Y, Jiang ZH, Mao EQ, Zou WG, Lei RQ, Han TQ, Zhang SD. Adenoviral transfer of human interleukin-10 gene in lethal pancreatitis. World J Gastroenterol 2004; 10(20): 3021-3025
- URL: https://www.wjgnet.com/1007-9327/full/v10/i20/3021.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i20.3021
The pathogenesis of severe acute pancreatitis (SAP) is complicated. Studies have indicated that the explosive production and release of pro-inflammatory cytokines play an important role in its pathogenesis. There is evidence that TNF-α and interleukin-1 (IL-1) are very important inflammatory cytokines in this process[1,2]. Although pro-inflammatory cytokines are necessary for protecting against inflammation in the early phase, uncontrolled and adverse inflammatory effects of the systemic cytokine response could also be harmful. Thus, inhibiting the synthesis of pro-inflammatory cytokines and altering the balance between pro- and anti- inflammatory cytokines might significantly affect the severity of pancreatitis and the survival rate[3,4]. IL-10 is a major anti-inflammatory cytokine. It has been shown to be down-regulated the expression of TNF and other inflammatory cytokines from activated macrophages. IL-10 could block the release of oxygen free radicals and nitrogen oxide. In consequence[5], it could decrease the mortality[6-8]. These findings suggested that administration of IL-10 could be useful in blocking these pro-inflammatory cytokines during the initiation of acute pancreatitis.
Gene therapy as a novel drug delivery system to express proteins in individual tissues has been used in inflammatory diseases[9]. The use of deficient adenovirus vectors might be a useful tool for this gene therapy due to its high infectious activity and high protein expression ability. Gene therapy has been proposed in many inflammation diseases to produce anti-inflammatory cytokines at local sites[10,11]. However, transfection of SAP rats with an adenoviral vector has not been previously reported.
In this paper, we reported a successful gene therapy for SAP rats. Our experiments showed that human IL-10 expression in tissues could block the development of SAP and improve the survival rate. The results suggest a novel potential therapeutic approach for the treatment of patients with acute pancreatitis.
Adenovirus expression system (Adenoviral Gateway Expression Kit, 293A Cell Line, Gateway LR Clonase Enzyme Mix, Lipofectamine 2000) and TRIzol reagent were provided by Invitrogen (San Diego, CA). pcDNA3IL-10 plasmid was generously donated by professor XY Liu (Shanghai Institute for Biological Sciences, Chinese Academy of Sciences, Shanghai, China). Restriction enzyme (HindIII, EcoRI, BamHI, SalI) was obtained from Promega (Madison, Wi, USA). Sodium taurocholate was purchased from Sigma Chemical Company (St Louis, MO). Amylase enzyme assay kits were obtained from Shanghai Kehua-Hualing Diagnosis Kit Co (Shanghai, China). An ELISA kit specific for human IL-10 was purchased from Diaclone Research International (BESANÇON Cedex, French).
Human IL-10 cDNA was cloned into the BamHI and EcoRI sites of pEntr 1A vector. Then the IL-10 cDNA was moved into the adenoviral destination vector from pEntr 1A vector by Gateway LR Clonase. The recombinant E1 deleted type 5 adenoviral vector encode hIL-10 under the transcriptional control of the cytomegalovirus promoter and contained the bGHp(A) sequence. Adenovirus hIL-10 vector was transfected into 293A human embryonic kidney cell line with Lipofectamine 2000. After transfection, cells were fed with fresh DMEM solution until the onset of cytopathic effect. Viruses were propagated in the 293A and purified by ultra centrifugation through two caesium chloride gradients. The titer of adenoviral vectors was determined by plaque assay on 293A cells. Viral stocks were aliquoted and stored in 100 mL/L glycerol at -80 °C until use.
Sprague-Dawley (SD) rats were purchased from Shanghai BK Experimental Animal Company (Shanghai, China) and maintained in filter-top cages under specific pathogen-free conditions. The Animal Study Ethics Committee of Shanghai Second Medical University approved all experiments. The experiments were conducted in 6-7 week-old male rats, weighing 170-180 g. Adult healthy male SD rats (n = 20) were given a single intraperitoneal injection of adenovirus PBS solution containing 1010 pfu of the AdvhIL-10. All injections were performed with a sterile 25-gauge needle in the left lower quadrant of the abdomen. Rats were anesthetized using sodium pentobarbital (40 mg/kg injection, intraperitoneal) and killed 24, 48, 96, and 168 h after transfection. Blood samples were obtained from the celiac artery. The samples were separated and stored at -70 °C for determination of amylase. Pancreas, liver and lung were immediately dissected from their attachments and divided for isolation of total RNA and total protein. some samples were fixed in 40 g/L buffered formaldehyde for histopathological test. PBS control group (n = 5) received an intraperitoneal injection of PBS solution, and the empty vector control group (n = 5) was given a single intraperitoneal injection of empty vector (Adv0) containing 1010 pfu viruses. Tissues of both control groups were harvested 24 h later as previously described.
SD rats (n = 80) were randomly divided into four groups: normal, AdvhIL-10, Adv0, and PBS groups. After laparotomy, SAP model was induced through injection of 0.5 mL of 30 g/L sodium taurocholate via the pancreatic duct. Twenty minutes after sodium taurocholate injection, AdvhIL-10 group received 0.5 mL single intraperitoneal injection of AdvhIL-10 containing 1010 pfu of AdvhIL-10. Two control groups received 0.5 mL single intraperitoneal injection of Adv0 (containing 1010 pfu viruses) or PBS solution, respectively. In each group, 5 rats were sacrificed by dislocation of the cervical vertebra 24 and 48 h after induction of SAP, respectively. Blood samples were obtained as previously described for determination of amylase. Pancreas, liver, and lungs were harvested as previously described and prepared for RT-PCR, ELISA, and histopathological test. Mortality was observed among the rats 7 d after the initiation of pancreatitis.
Serum amylase levels were measured at 37 °C by an enzymatic assay using a Beckman nucleic acid and protein analyzer DU 640 (BECKMAN, USA) standardized for these rat proteins. All serum samples were assayed in duplicate, and the results were averaged at the end of the experiment. Rat TNF-α , hIL-10 levels in the homogenates were determined using commercially available enzyme-linked immunosorbent assay (ELISA) kits from Diaclone Research International.
Total RNA was extracted from homogenized liver, pancreas, and lung with TRIzol reagent following the manufacturer’s instructions. Aliquots of 5 μg of total RNA were reverse-transcribed by using a first-strand cDNA synthesis kit (Promega A3500). The cDNA was then amplified by polymerase chain reaction using primers specific for hIL-10 or TNF-α with β - actin primers serving as controls. The primer sequences and sizes of amplification products were as follows: hIL-10 sense, 5’- GAGCG, GATCC, ATGAA, GTGGG, TAACC, TTTC-3’; antisense, 5’- ATACG, AATTC, CTGCA, GCGGC, CGCCA, CT-3’ (540 base pairs); TNF sense, 5’-TGCCT, CAGCC, TCTTC, TCATT-3’; antisense, 5’-ACACC, CATTC, CCTTC, ACAGA-3’ (446 base pairs); β -actin sense, 5’-TTGTA, ACCAA, CCTGGG, ACGAT, ATGG-3’; antisense, 5’-TGGAA, GACTC, CTCCC, AGGTA-3’ (515 base pairs). Following an initial denaturation at 94 °C for 5 min, the samples were amplified by 27 cycles of denaturation at 94 °C for 40 s, annealing at 58 °C for 50 s, extension at 72 °C for 90 s, and ended by extension at 72 °C for 15 min. The PCR reaction products were separated on 20 g/L agarose gels. Photo-micrographs of ethidium bromide stained gels were taken. Relative mRNA levels of hIL-10, TNF-α and β -actin were determined by computer-assisted densitometric scanning.
Pancreas samples were fixed in 40 g/L buffered formaldehyde and embedded in paraffin.Then the samples were cut into 5 μm thick sections,and stained with haematoxylin and eosin for light microscropic examination. Histological assessment was performed by an investigator blinded to the treatment group. The severity of pancreatitis was determined by the degree of edema, hemorrhage, inflammation and necrosis[11].
Results were expressed as mean ± SD. Differences between groups were compared by two-way ANOVA. P < 0.05 was considered statistically significant. Mortality was assessed by Kaplan-Meier and log rank analysis. All data processing was done with a statistical program, SPSS 11.0.
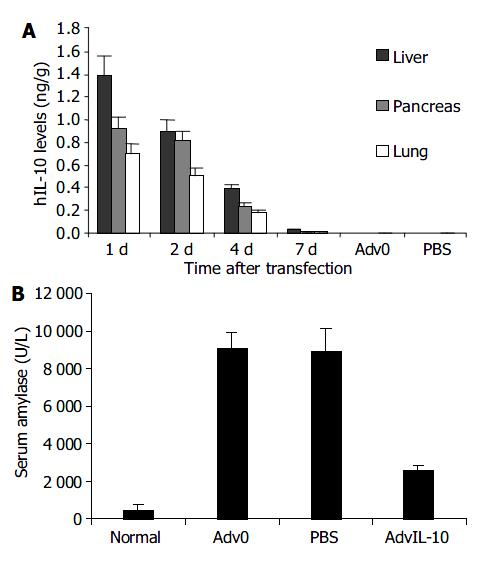
Twenty-four h after transfection, The normal and two control groups did not show hIL-10 protein expression.
hIL-10 protein was effectively measured in healthy rats after AdvhIL-10 administration. The hIL-10 level in pancreas, liver, and lung increased and reached its peak at 24 h post-transfection, then decreased gradually 4 d after transfection. The hIL-10 level in the tissues was undetectable 7 d post-transfection, and was the highest in the liver among the three observed organs (Figure 1A).
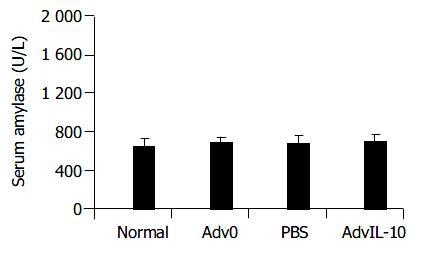
There were no significant differences in serum amylase level among the rats effectively transfected with AdvIL-10 and Adv0 and PBS treated rats in the duration of the experiment (Figure 2, P > 0.05). In addition, no detectable histology alternation of the pancreas, liver, and lungs was observed in the transfected rats. Panceatic edema, hyperemia, exudation and necrosis were not detected. These findings indicated that adenovirus gene transfer could be performed safely.
The SAP rats treated with Adv0 and PBS showed a significant increase in serum amylase level compared to normal rats (P < 0.05). Transfection with AdvhIL-10 after induction of pancreatitis decreased the severity of SAP, as evidenced by markedly attenuated amylase production compared to the two control SAP groups (Figure 1B, P < 0.05).
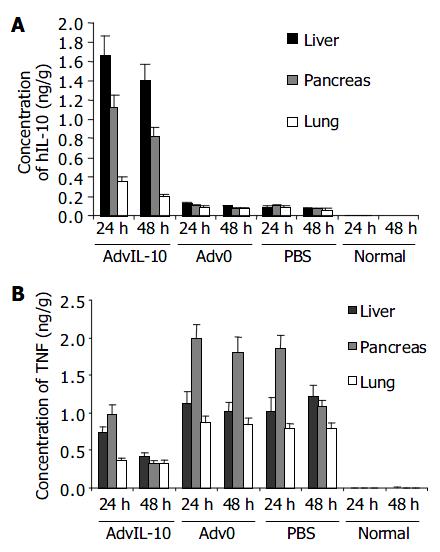
HIL-10 mRNA was strongly expressed in the AdvhIL-10 treated pancreatitis rats 24 h post-tranfection. The levels of hIL-10 in pancreas, liver, and lung did not show significant difference. A weak expression of hIL-10 was observed in Adv0 and PBS treated pancreatitis rats 24 h post-tranfection. TNF-α gene in the normal rats was not detected. The expression of TNF-α gene was significantly higher in the pancreatitis rats treated with Adv0 and PBS than that in those treated with AdvhIL-10 (P < 0.05, Figure 3). In the AdvhIL-10 group, the level of TNF-α gene was moderate in pancreas and weak in the liver and lung (Figure 4).
The levels of IL-10 in pancreas, liver and lung in the AdvhIL-10 group were markedly higher than those in the two control groups 24 and 48 h post-transfection (P < 0.05). No significant difference was observed between the two control groups (Figure 4A). In normal rats, TNF-α expression was undetectable. Twenty-four h after induction of pancreatitis, TNF-α level was significantly higher in Adv0 and PBS groups than that in AdvIL-10 group (P < 0.05, Figure 4B).
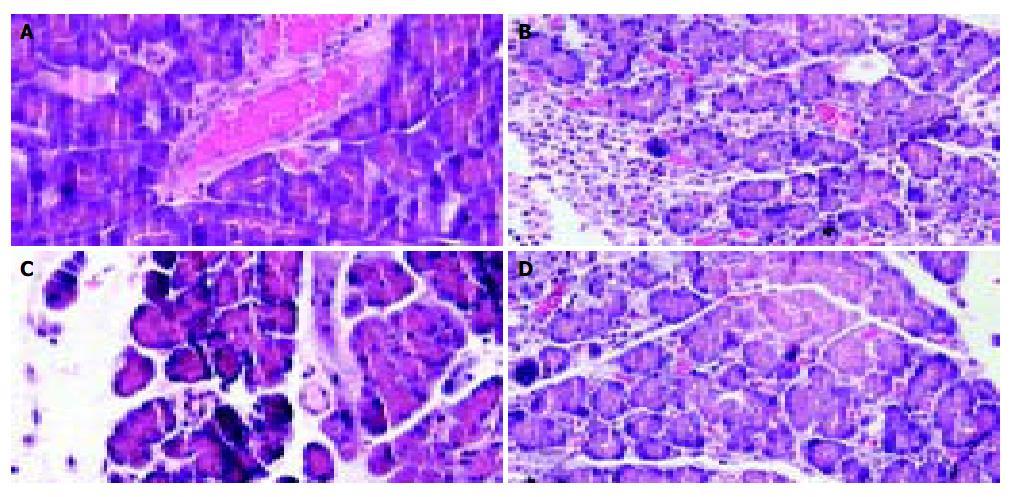
Hematoxylin and eosin-stained sections of pancreas from pancreatitis rats treated with Adv0 and PBS showed an increase in inflammation, hemorrhage, and necrosis after injection of sodium taurocholate. The histological changes in the AdvIL-10 treated pancreatitis rats were mild compared with the control groups. Most of the pathological parameters of pancreatic injury were significantly decreased in pancreatitis rats transfected with AdvIL-10 gene compared to the pancreatitis rats treated with Adv0 and PBS (P < 0.05, Table 1, Figure 5).
| Normal | PBS (or Adv0) | hIL-10 transfected | |
| Edema | 0 ± 0 | 2.26 ± 0.13 | 1.74 ± 0.13 |
| Necrosis | 0 ± 0 | 2.56 ± 0.21 | 0.51 ± 0.13 |
| Inflammation | 0 ± 0 | 3.43 ± 0.14 | 0.46 ± 0.14 |
| Hemorrhage | 0 ± 0 | 2.31 ± 0.17 | 0.13 ± 0.11 |
No significant difference in mortality was observed between the two control SAP groups within 7 d after SAP inducetion, 90% of the rats were died in the Adv0 and PBS treated groups, while only 20% of the rats were died in the AdvIL-10 gene therapy group (Figure 6).
IL-10 gene therapy has been widely explored for the treatment of many diseases, including intestinal transplantation immune regulation, bronchiolitis, endotoxemia, and rheumatoid arthritis[12-15]. In the present report, we explored the tissue levels, distribution, and biological responses of hIL-10 mediated by adenoviral vectors in healthy and pancreatitis rats. Adenovirus vector has many advantages. Adenovirus genomes did not integrate into the host cell chromosome and had a high efficiency gene transfer than cationic liposome regardless of the proliferative state of tissues. Although the duration of gene expression was short, the level of therapeutic gene expression was much higher [16]. Adenoviral vectors could bind to cell surface integrins and gain entry by receptor mediated endocytosis using receptors such as Coxsackie virus and adenovirus receptor[17]. Thus, adenoviral vector could offer the opportunity to target specific tissues for highly local expression, owing to special tropism for pulmonary epithelial cells, hepatocytes, and pancreatic epithelial cells[18-20]. Expression of adenoviral vector was rapid, protein appearance usually occurred within hours and peak concentration appeared within 1-2 d after adenoviral administration[21]. Furthermore, adenovirus vectors could be prepared at a much higher titre.
Norman James[22] reported that with very few exceptions, cytokines were not constitutively produced. The results of our experiment is consistent with Norman James’s results. We explored the use of adenovirus based gene therapy to deliver hIL-10 gene intraperitoneally to healthy and pancreatitis rats. Tissue hIL-10 protein was highly produced 24, 48 h after AdvhIL-10 was injected into peritoneal cavity of healthy rats. The current study demonstrated that the current adenovirus delivery system could offer a highly efficient transfection and well-correlated tissue accummulation. We also found that highly efficient transfection with AdvhIL-10 did not result in an increase in amylase and lipase or any alteration in pancreatic histopathology. Therefore, adenoviral vector mediated hIL-10 gene delivery in healthy rats was well tolerable and safe. In SAP rats, AdvhIL-10 attenuated the release of serum amylase and decreased histologic injury significantly. In addition, the action of TNF-α could also be diminished by IL-10. The important factor of our experiment was the increase of survival rate resulted from adenoviral vector mediated hIL-10 gene delivery in rats with lethal acute pancreatitis. These results are consistent with several previous reports on adenoviral vector-mediated gene therapy in pancreatitis and acute lung inflammation[9,23,24].
There is sufficient evidence (immunological, pathophysiolo-gical, and biochemical) that SAP is a systemic rather than a local critical condition. The severity of the process varied from a limited local inflammation of the pancreas to a systemic multi-organ failure. SAP was characterised by enzyme activation, interstitial edema, hemorrhage and necrosis[25]. Many pro- and anti- inflammation cytokines are involved in the initiation of acute pancreatitis. IL-10, produced by TH2 cells, macrophages, stellate cells and hepatocytes, has been reported to play an important role in inflammatory diseases[26]. It has been reported the deficiency of IL-10 gene could prompte colitis and fibrosis probably by its failure in inhibiting the overproduction of tumor growth factor-β1 and TNF[27,28]. The later is secreted by macrophages and can enhance inflammation in a local site. In animals, IL-10 is endogenously released during inflammatory diseases, and its blockade could result in higher elevations of TNF as well as more severe histologic injury[10]. Production of large quantities of exogenous IL-10 in local sites of inflammation could change the balance of pro- and anti- inflammation cytokines and block the production and release of TNF and other pro-inflammation cytokines. IL-10 adminstration could improve of local and systemic conditions. In the current study, exogenous hIL-10 was found to be able to inhibit the progress of acute pancreatitis in rats. Similar results were also reported by previous studies[10,11].
The time to administrate AdvIL-10 seems to be very important, since it takes time to transfect and express protein. Thus, early administration of AdvIL-10 might block the induction of TNF and IL-1[5]. Kato et al reported that the time to administratie hIL-10 should be in the early period of sepsis. In the current study, hIL-10 gene was administrated 20 min after the induction of SAP, and the highest level of IL-10 protein was observed 24 h after adminstration, which was very similar to a previous study by Denham et al[10].
In conclusion, human genes can be effectively transfected into rat pancreas, livers, and lungs using a adenoviral vector-mediated delivery system. Transfection of hIL-10 gene can decrease the severity of pancreatitis and improve the survival rate. As gene therapy is becoming a more acceptable method of treatment, it is anticipated that adenovirus-based gene therapy will become available as a drug delivery system. Cytokine modulating therapies, like IL-10, represent an attractive therapeutic approach for the treatment of acute pancreatitis patients in clinic.
Edited by Kumar M and Wang XL Proofread by Xu FM
| 1. | Viedma JA, Pérez-Mateo M, Domínguez JE, Carballo F. Role of interleukin-6 in acute pancreatitis. Comparison with C-reactive protein and phospholipase A. Gut. 1992;33:1264-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 114] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Grewal HP, Mohey el Din A, Gaber L, Kotb M, Gaber AO. Amelioration of the physiologic and biochemical changes of acute pancreatitis using an anti-TNF-alpha polyclonal antibody. Am J Surg. 1994;167:214-28; discussion 214-28;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 142] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Walley KR, Lukacs NW, Standiford TJ, Strieter RM, Kunkel SL. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun. 1996;64:4733-4738. [PubMed] |
| 4. | Weiss YG, Deutschman CS. Modulation of gene expression in critical illness: a new millennium or a brave new world? Crit Care Med. 2000;28:3078-3079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Rongione AJ, Kusske AM, Kwan K, Ashley SW, Reber HA, McFadden DW. Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology. 1997;112:960-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 224] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Gazzinelli RT, Oswald IP, James SL, Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J Immunol. 1992;148:1792-1796. [PubMed] |
| 7. | Pradier O, Gérard C, Delvaux A, Lybin M, Abramowicz D, Capel P, Velu T, Goldman M. Interleukin-10 inhibits the induction of monocyte procoagulant activity by bacterial lipopolysaccharide. Eur J Immunol. 1993;23:2700-2703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Howard M, Muchamuel T, Andrade S, Menon S. Interleukin 10 protects mice from lethal endotoxemia. J Exp Med. 1993;177:1205-1208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 578] [Cited by in RCA: 583] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 9. | Minter RM, Ferry MA, Murday ME, Tannahill CL, Bahjat FR, Oberholzer C, Oberholzer A, LaFace D, Hutchins B, Wen S. Adenoviral delivery of human and viral IL-10 in murine sepsis. J Immunol. 2001;167:1053-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Denham W, Denham D, Yang J, Carter G, MacKay S, Moldawer LL, Carey LC, Norman J. Transient human gene therapy: a novel cytokine regulatory strategy for experimental pancreatitis. Ann Surg. 1998;227:812-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Zou WG, Wang DS, Lang MF, Jin DY, Xu DH, Zheng ZC, Wu ZH, Liu XY. Human interleukin 10 gene therapy decreases the severity and mortality of lethal pancreatitis in rats. J Surg Res. 2002;103:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Zhu M, Wei MF, Liu F, Shi HF, Wang G. Interleukin-10 modified dendritic cells induce allo-hyporesponsiveness and prolong small intestine allograft survival. World J Gastroenterol. 2003;9:2509-2512. [PubMed] |
| 13. | Boehler A, Chamberlain D, Xing Z, Slutsky AS, Jordana M, Gauldie J, Liu M, Keshavjee S. Adenovirus-mediated interleukin-10 gene transfer inhibits post-transplant fibrous airway obliteration in an animal model of bronchiolitis obliterans. Hum Gene Ther. 1998;9:541-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Xing Z, Ohkawara Y, Jordana M, Graham FL, Gauldie J. Adenoviral vector-mediated interleukin-10 expression in vivo: intramuscular gene transfer inhibits cytokine responses in endotoxemia. Gene Ther. 1997;4:140-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Whalen JD, Lechman EL, Carlos CA, Weiss K, Kovesdi I, Glorioso JC, Robbins PD, Evans CH. Adenoviral transfer of the viral IL-10 gene periarticularly to mouse paws suppresses development of collagen-induced arthritis in both injected and uninjected paws. J Immunol. 1999;162:3625-3632. [PubMed] |
| 16. | Wickham TJ. Targeting adenovirus. Gene Ther. 2000;7:110-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 195] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2385] [Cited by in RCA: 2318] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 18. | Crystal RG. The gene as the drug. Nat Med. 1995;1:15-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 98] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Worgall S, Wolff G, Falck-Pedersen E, Crystal RG. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum Gene Ther. 1997;8:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 381] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 20. | Weber M, Deng S, Kucher T, Shaked A, Ketchum RJ, Brayman KL. Adenoviral transfection of isolated pancreatic islets: a study of programmed cell death (apoptosis) and islet function. J Surg Res. 1997;69:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Minter RM, Rectenwald JE, Fukuzuka K, Tannahill CL, La Face D, Tsai V, Ahmed I, Hutchins E, Moyer R, Copeland EM. TNF-alpha receptor signaling and IL-10 gene therapy regulate the innate and humoral immune responses to recombinant adenovirus in the lung. J Immunol. 2000;164:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Norman J. The role of cytokines in the pathogenesis of acute pancreatitis. Am J Surg. 1998;175:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 509] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 23. | Van Laethem JL, Marchant A, Delvaux A, Goldman M, Robberecht P, Velu T, Devière J. Interleukin 10 prevents necrosis in murine experimental acute pancreatitis. Gastroenterology. 1995;108:1917-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 164] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Minter RM, Ferry MA, Rectenwald JE, Bahjat FR, Oberholzer A, Oberholzer C, La Face D, Tsai V, Ahmed CM, Hutchins B. Extended lung expression and increased tissue localization of viral IL-10 with adenoviral gene therapy. Proc Natl Acad Sci U S A. 2001;98:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Jungermann J, Lerch MM, Weidenbach H, Lutz MP, Krüger B, Adler G. Disassembly of rat pancreatic acinar cell cytoskeleton during supramaximal secretagogue stimulation. Am J Physiol. 1995;268:G328-G338. [PubMed] |
| 26. | de Vries JE. Immunosuppressive and anti-inflammatory properties of interleukin 10. Ann Med. 1995;27:537-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 286] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 27. | Lindsay JO, Ciesielski CJ, Scheinin T, Hodgson HJ, Brennan FM. The prevention and treatment of murine colitis using gene therapy with adenoviral vectors encoding IL-10. J Immunol. 2001;166:7625-7633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Thompson K, Maltby J, Fallowfield J, McAulay M, Millward-Sadler H, Sheron N. Interleukin-10 expression and function in experimental murine liver inflammation and fibrosis. Hepatology. 1998;28:1597-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 189] [Article Influence: 7.0] [Reference Citation Analysis (0)] |