Published online Oct 15, 2004. doi: 10.3748/wjg.v10.i20.2927
Revised: November 22, 2003
Accepted: December 8, 2003
Published online: October 15, 2004
AIM: Recombined plasmid pETNF-P16 was constructed to investigate its expression properties in esophageal squamous carcinoma cell line EC9706 induced by X-ray irradiation and the feasibility of gene-radiotherapy for esophageal carcinoma.
METHODS: Recombined plasmid pETNF-P16 was constructed and transfected into EC9706 cells with lipofectamine. ELISA, Western blot, and immunocytochemistry were performed to determine the expression properties of pETNF-P16 in EC9706 after transfection induced by X-ray irradiation.
RESULTS: Eukaryotic expression vector pETNF-P16 was successfully constructed and transfected into EC9706 cells. TNFα expressions were significantly increased in the transfected cells after different doses of X-ray irradiation than in those after 0Gy irradiation (1192.330-2026.518 pg/mL, P < 0.05-0.01), and the TNFα expressions and P16 were significantly higher 6-48 h after 2 Gy X-ray irradiation (358.963-585.571 pg/mL, P < 0.05-0.001). No P16 expression was detected in normal EC9706 cells. However, there was strong expression in the transfected and irradiation groups.
CONCLUSION: X-ray irradiation induction could significantly enhance TNFα and P16 expression in EC9706 cells transfected with pETNF-P16 plasmid. These results may provide important experimental data and therapeutic potential for gene-radiotherapy of esophageal carcinoma.
- Citation: Wu CM, Huang TH, Xie QD, Wu DS, Xu XH. Construction of pETNF-P16 plasmid and its expression properties in EC9706 cell line induced by X-ray irradiation. World J Gastroenterol 2004; 10(20): 2927-2930
- URL: https://www.wjgnet.com/1007-9327/full/v10/i20/2927.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i20.2927
Esophageal carcinoma is one of the most frequent malignant tumors. Its treatment choices include surgical resection, radiation, chemotherapy and biological therapy. However, their limitations and adverse effects influence the therapeutic results. In 1992, Wechselbaum proposed the theory of gene-radiation combination treatment, that is, to ligate the promoter with irradiation-induced function and treatment gene so as to take advantages of the combined therapeutic functions of both modalities[1].
In this study, pETNF-P16 plasmid was constructed and transfected into human esophageal cancer cell line EC9706. The expressions of TNFα and P16 in the transfected cells exposed to different doses of X-ray irradiation and the time course of the expressions after 2Gy X-ray irradiation were detected to explore the feasibility of gene-radiotherapy for esophageal carcinoma.
The EC9706 was maintained in Dulbecco’s modified Eagle’s medium (DMEM), high glucose media (Life Technologies) and generously supplemented with 100 mL/L fetal bovine serum (Hyclone Laboratories), penicillin, streptomycin and nonessential amino acids (Life Technologies). PIRES1 vector was bought from Promega-Biotec (Shanghai, Promega Corporation).
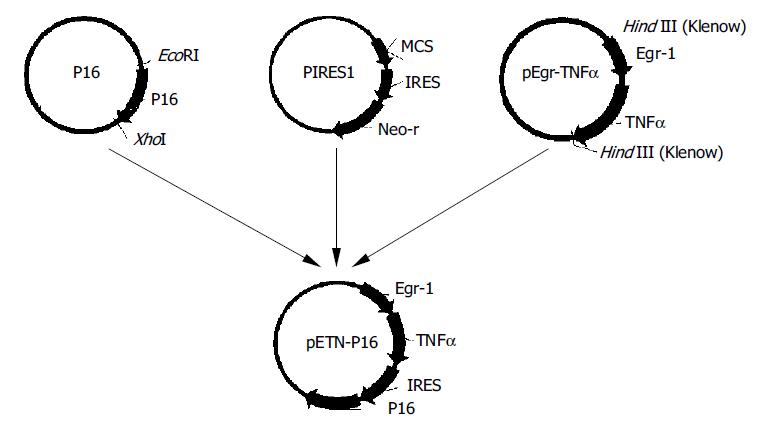
The expression vector for TNFα and P16 was constructed as Figure 1.
Transfection of EC9706 cells was carried out in a 6-well plate. The transfection procedure began when the cells reached 70% confluence on the surface of plate wells. Solution A was prepared by separate addition of 10 µg of pETNF-P16 or PIRES1 to 100 µL serum-free medium (SFM), and solution B by addition of 10 µL liposome to 100 µL SFM. Solutions A and B were combined at room temperature for 30 min then 0.8 mL SFM was added to the tube containing the above solutions, and then the mixture was added to rinsed cells. The medium was replaced with a fresh and complete medium after 6 h in transfection. The cells were exposed to irradiation after 36 h in transfection.
TNFα protein was detected using ELISA kit (Genzyme). P16 protein was studied using Western blot analysis and immunocytochemistry (P16 antibody, Boster).
X-rays of 180 kV and 12 mA with 0.25 mmCu and 1.08 mm Al as filter were given at a dose-rate of 0.8639 Gy/min for doses of 2-20 Gy.
Student’s t test was used to determine the difference between groups. P values of less than 0.05 were considered statistically significant.
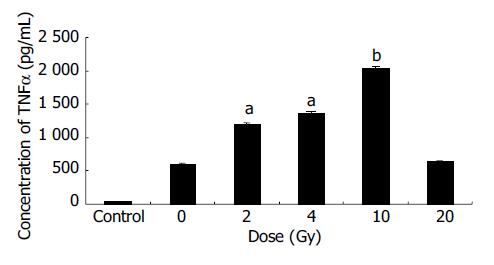
EC9706 cells transfected with pETNF-P16 received different doses of X-ray irradiation. The cells of control group were transfected with PIRES1. Eight hours after irradiation, the protein was extracted and TNFα expression was detected by ELISA.
The results showed that TNFα expression in the 2, 4, 10 Gy groups was significantly higher than that in 0 Gy group (P < 0.05-0.01) (Figure 2).
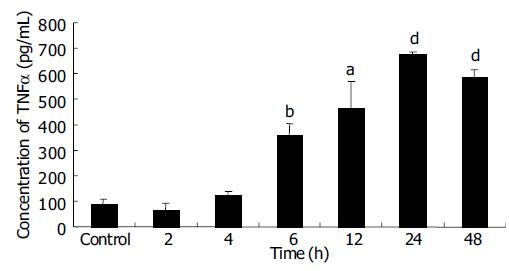
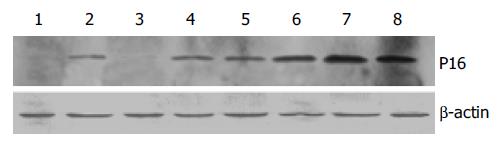
EC9706 cells transfected with pETNF-P16 received 2 Gy of X-ray irradiation while the control group did not receive. Proteins of TNFα and P16 were isolated at different time points after irradiation and detected by ELISA and Western blot.
ELISA results showed that TNFα expression increased from 2 to 24th, and reached the peak level at the 24th h, about 7.5 times of control group (P < 0.01). TNFα expression in 48 h group was significantly higher than that in control group (P<0.001), but lower than that in 24 h group (P > 0.05) (Figure 3).
The results of Western blot analysis showed no P16 expression in cells transfected with PIRES1 plasmid. After 2Gy X-ray irradiation, P16 expression in the cells transfected with pETNF-P16 plasmid increased between 2 h and 48 h. The expression in the control group was lower than that in 4 h to 48 h groups (Figure 4).
Thirty-six hours after transfected with pETNF-P16 plasmids, EC9706 cells received 2 Gy X-ray irradiation while the control group did not receive. After 24 h, anti-P16 IgG and Biotin-labeled secondary antibody were used to detect the P16 expression in treated cells. P16 protein was positive in the nucleus, cytoplasm and cellular membrane as judged by the brown color.
Immunocytochemical analysis demonstrated that all the normal EC9706 cells had no P16 expression. After transfected with pETNF-P16 plasmid, positive P16 expression in EC9706 cells was detected. Then, the transfected cells received 2 Gy X-ray irradiation and P16 expression remained positive, but morphological changes of EC9706 cells occurred.
It was reported that Egr-1 was transcriptionally induced following exposure to irradiation[2]. Sequences responsive to ionizing radiation-induced signals were determined by deletion analysis of the Egr-1 promoter. The results demonstrated that induction of Egr-1 by X-rays was conferred by serum response or CC (A/T) rGG elements. Further analysis confirmed that the region encompassing the three distal or upstream CC(A/T)rGG elements was functional in X-ray response[3-5].
Weichselbaum et al[6] were forerunners in tumor gene-radiotherapy. In 1992 they put forward that ionizing radiation could be used to activate the transcription of exogenous genes encoding cytotoxic proteins, and established the techniques that might be used to target gene therapy during the treatment of human neoplasms.
Combination of gene therapy with radiation therapy could overcome some problems and side effects of either radiation or gene therapy alone, including radioresistance of some tumors and toxicities to normal tissues. Weichselbaum et al[7] linked DNA sequences from the promotor region of Egr-1 to a cDNA sequence that encoded human tumor necrosis factor (TNF) alpha. Egr-TNF construction was transfected into a human cell line of hematopoietic origin, HL525 (clone 2). The latter was injected into human xenografts of the radioresistant human squamous cell carcinoma cell line SQ-20B. Animals treated with radiation and clone 2 demonstrated an improvement compared with those treated with radiation or injections of clone 2 alone. Thereafter, a variety of downstream genes were introduced to Egr-1 promoter to treat different tumors and similar results were obtained[8-10].
TNF has complex in-vivo anti-tumor actions and involves a series of biochemical reactions, and varies according to host metabolism. It could not only kill tumor cells directly, but also act indirectly by occluding the feeding vessels of tumors, promoting inflammation reaction of the host, stimulating cytotoxic activities of megalocytes and producing tumor-specific cytotoxic antibody. Up to now, TNF has been found to have the strongest direct tumor-killing action. However, severe side effects made the effects of systemic treatment unsatisfactory. By contrast, regional administration into the tumor had better therapeutic effects. It has been widely reported that intra-tumor TNF injection had no severe side effects on patients with malignant tumors[11-12]. We constructed pEgr-TNFα plasmid and injected it into mouse melanoma locally to induce its expression by X-ray irradiation. The results showed that pEgr-TNFα gene in combination with radiotherapy could significantly inhibit tumor and had no side effects[13].
P16 is a tumor suppressor gene product. Serrano et al[14] demonstrated that P16 bound to CDK4 and inhibited the catalytic activity of CDK4/cyclin D enzymes. P16 seemed to act in a regulatory feedback circuit with CDK4, D-type cyclins and retinoblastoma protein. Overexpression of P16 gene could block cell cycle progression through the G1-to-S phase boundary in a pRB-dependent manner[15,16]. Many P16 mutants identified from human tumors have been shown to have defects in this activity[17-19]. These suggested that the CDK4-inhibitory activity of p16 was involved in regulating cell cycle progression through the G1/S boundary.
On the basis of anti-tumor function of TNFα and P16, we constructed pETNF-P16 plasmid and transfected EC9706 cells to investigate the expression properties of plasmid induced by X-ray irradiation. The results showed that TNFα expression in pETNF-P16-transfected cells induced by irradiation was higher than that in control group (P < 0.05-0.01). Time-course studies between 6 h and 48 h after 2Gy irradiation revealed that TNFα expression in X-ray induced groups was higher than that in the control group (P < 0.05-0.001). It gradually increased and peaked at the 24th h with the expression level of 7.5 times of control group (P < 0.01). Immunocytochemistry showed no P16 expression in the control group, but strong expressions in the transfected and irradiated group. However, the cellular morphology was altered in the latter group and the mechanism is to be clarified.
Esophageal carcinoma still has high morbidity and mortality in China[20-22], and its treatment is still difficult[23-30]. Our work might have laid some theoretical basis for further study on esophageal cancer gene-radiotherapy, which should have promising therapeutic potential.
We are very grateful to Professor Ming-Rong Wang, Institute of Cancer, Chinese Academy of Medical Sciences for his kindness in providing us with the EC9706 cell line.
Edited by Wang XL and Xu FM
| 1. | Weichselbaum RR, Hallahan DE, Beckett MA, Mauceri HJ, Lee H, Sukhatme VP, Kufe DW. Gene therapy targeted by radiation preferentially radiosensitizes tumor cells. Cancer Res. 1994;54:4266-4269. [PubMed] |
| 2. | Christy B, Nathans D. DNA binding site of the growth factor-inducible protein Zif268. Proc Natl Acad Sci U S A. 1989;86:8737-8741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 419] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 3. | Sukhatme VP. Early transcriptional events in cell growth: the Egr family. J Am Soc Nephrol. 1990;1:859-866. [PubMed] |
| 4. | Cao XM, Koski RA, Gashler A, McKiernan M, Morris CF, Gaffney R, Hay RV, Sukhatme VP. Identification and characterization of the Egr-1 gene product, a DNA-binding zinc finger protein induced by differentiation and growth signals. Mol Cell Biol. 1990;10:1931-1939. [PubMed] |
| 5. | Tsai-Morris CH, Cao XM, Sukhatme VP. 5' flanking sequence and genomic structure of Egr-1, a murine mitogen inducible zinc finger encoding gene. Nucleic Acids Res. 1988;16:8835-8846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 128] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Weichselbaum RR, Kufe DW, Advani SJ, Roizman B. Molecular targeting of gene therapy and radiotherapy. Acta Oncol. 2001;40:735-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Datta R, Taneja N, Sukhatme VP, Qureshi SA, Weichselbaum R, Kufe DW. Reactive oxygen intermediates target CC(A/T)6GG sequences to mediate activation of the early growth response 1 transcription factor gene by ionizing radiation. Proc Natl Acad Sci U S A. 1993;90:2419-2422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 124] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Hanna NN, Seetharam S, Mauceri HJ, Beckett MA, Jaskowiak NT, Salloum RM, Hari D, Dhanabal M, Ramchandran R, Kalluri R. Antitumor interaction of short-course endostatin and ionizing radiation. Cancer J. 2000;6:287-293. [PubMed] |
| 9. | Takahashi T, Namiki Y, Ohno T. Induction of the suicide HSV-TK gene by activation of the Egr-1 promoter with radioisotopes. Hum Gene Ther. 1997;8:827-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Griscelli F, Li H, Cheong C, Opolon P, Bennaceur-Griscelli A, Vassal G, Soria J, Soria C, Lu H, Perricaudet M. Combined effects of radiotherapy and angiostatin gene therapy in glioma tumor model. Proc Natl Acad Sci U S A. 2000;97:6698-6703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Vilcek J, Lee TH. Tumor necrosis factor. New insights into the molecular mechanisms of its multiple actions. J Biol Chem. 1991;266:7313-7316. [PubMed] |
| 12. | Rothe J, Gehr G, Loetscher H, Lesslauer W. Tumor necrosis factor receptors--structure and function. Immunol Res. 1992;11:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 115] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Wu CM, Li XY, Liu SZ. Construction of pEgr.p-TNFα and its expression in NIH3T3 cells induced by ionizing irradiation. Chin J Radiol Med Prot. 2001;21:332-334. |
| 14. | Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2515] [Cited by in RCA: 2557] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 15. | Koh J, Enders GH, Dynlacht BD, Harlow E. Tumour-derived p16 alleles encoding proteins defective in cell-cycle inhibition. Nature. 1995;375:506-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 378] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 16. | Lukas J, Parry D, Aagaard L, Mann DJ, Bartkova J, Strauss M, Peters G, Bartek J. Retinoblastoma-protein-dependent cell-cycle inhibition by the tumour suppressor p16. Nature. 1995;375:503-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 652] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 17. | Monzon J, Liu L, Brill H, Goldstein AM, Tucker MA, From L, McLaughlin J, Hogg D, Lassam NJ. CDKN2A mutations in multiple primary melanomas. N Engl J Med. 1998;338:879-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 178] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Nobori T, Miura K, Wu DJ, Lois A, Takabayashi K, Carson DA. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994;368:753-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1190] [Cited by in RCA: 1166] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 19. | Soufir N, Avril MF, Chompret A, Demenais F, Bombled J, Spatz A, Stoppa-Lyonnet D, Bénard J, Bressac-de Paillerets B. Prevalence of p16 and CDK4 germline mutations in 48 melanoma-prone families in France. The French Familial Melanoma Study Group. Hum Mol Genet. 1998;7:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 264] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 20. | Zhao XJ, Li H, Chen H, Liu YX, Zhang LH, Liu SX, Feng QL. Expression of e-cadherin and beta-catenin in human esophageal squamous cell carcinoma: relationships with prognosis. World J Gastroenterol. 2003;9:225-232. [PubMed] |
| 21. | Heidecke CD, Weighardt H, Feith M, Fink U, Zimmermann F, Stein HJ, Siewert JR, Holzmann B. Neoadjuvant treatment of esophageal cancer: Immunosuppression following combined radiochemotherapy. Surgery. 2002;132:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Tsunoo H, Komura S, Ohishi N, Yajima H, Akiyama S, Kasai Y, Ito K, Nakao A, Yagi K. Effect of transfection with human interferon-beta gene entrapped in cationic multilamellar liposomes in combination with 5-fluorouracil on the growth of human esophageal cancer cells in vitro. Anticancer Res. 2002;22:1537-1543. [PubMed] |
| 23. | Nemoto K, Zhao HJ, Goto T, Ogawa Y, Takai Y, Matsushita H, Takeda K, Takahashi C, Saito H, Yamada S. Radiation therapy for limited-stage small-cell esophageal cancer. Am J Clin Oncol. 2002;25:404-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Tachibana M, Dhar DK, Kinugasa S, Yoshimura H, Fujii T, Shibakita M, Ohno S, Ueda S, Kohno H, Nagasue N. Esophageal cancer patients surviving 6 years after esophagectomy. Langenbecks Arch Surg. 2002;387:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Wilson KS, Wilson AG, Dewar GJ. Curative treatment for esophageal cancer: Vancouver Island Cancer Centre experience from 1993 to 1998. Can J Gastroenterol. 2002;16:361-368. [PubMed] |
| 26. | Liu HH, Yoshida M, Momma K, Oohashi K, Funada N. Detection and treatment of an asymptomatic case of early esophageal cancer using chromoendoscopy and endoscopic mucosal resection. J Formos Med Assoc. 2002;101:219-222. [PubMed] |
| 27. | Wang AH, Sun CS, Li LS, Huang JY, Chen QS. Relationship of tobacco smoking CYP1A1 GSTM1 gene polymorphism and esophageal cancer in Xi'an. World J Gastroenterol. 2002;8:49-53. [PubMed] |
| 28. | Muto M, Ohtsu A, Miyata Y, Shioyama Y, Boku N, Yoshida S. Self-expandable metallic stents for patients with recurrent esophageal carcinoma after failure of primary chemoradiotherapy. Jpn J Clin Oncol. 2001;31:270-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Yeh AM, Mendenhall WM, Morris CG, Zlotecki RA, Desnoyers RJ, Vogel SB. Factors predictive of survival for esophageal carcinoma treated with preoperative radiotherapy with or without chemotherapy followed by surgery. J Surg Oncol. 2003;83:14-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Lew JI, Gooding WE, Ribeiro U, Safatle-Ribeiro AV, Posner MC. Long-term survival following induction chemoradiotherapy and esophagectomy for esophageal carcinoma. Arch Surg. 2001;136:737-42; discussion 743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |