Published online Oct 1, 2004. doi: 10.3748/wjg.v10.i19.2878
Revised: April 1, 2004
Accepted: April 5, 2004
Published online: October 1, 2004
AIM: To investigate the expression level of plasma vascular endothelial growth factor (P-VEGF) in patients with hepatocellular carcinoma (HCC) and its relationship with the clinicopathologic characteristics, and to examine the changes of P-VEGF in the course of transcatheter arterial chemoembolization (TACE).
METHODS: Peripheral blood samples were taken from 45 HCC patients before and 1, 3, 7 d, and 1 mo after TACE. Plasma VEGF level was measured with the quantitative sandwich enzyme-linked immunosorbent assay (ELISA). Twenty patients with benign liver lesions and 17 healthy control subjects were also included in this study.
RESULTS: Plasma VEGF levels in HCC patients were significantly elevated as compared to those in patients with benign liver lesions (P = 0.006) and in the normal controls (P = 0.003). Significant differences were observed when P-VEGF was categorized by tumor size (P = 0.006), portal vein thrombosis (P = 0.011), distant metastasis (P = 0.017), arterial-portal vein shunting (P = 0.026), and International Union Against Cancer (UICC) TNM stage (P = 0.044). There was no correlation between plasma level of VEGF and the level of alpha fetoprotein (α-FP) (r = 0.068, P = 0.658) and weakly correlated with the number of platelets (r = 0.312, P = 0.038). P-VEGF levels increased significantly and reached the peak value on the first day after TACE, and then decreased gradually. The change rate of P-VEGF concentration (one month post-TACE/pre-TACE × 100%) was correlated with the retention rate of lipiodol oil (rs = 0.494, P = 0.001) and the tumor volume change (rs = 0.340, P = 0.034). The patients who achieved a partial or complete response to TACE therapy showed significantly less pre-treatment P-VEGF than those nonresponders (P = 0.025). A high pre-therapeutic P-VEGF level was associated with poor response to treatment (P = 0.018).
CONCLUSION: A high pre-treatment P-VEGF level is a useful marker for tumor progression, especially for vascular invasion. TACE increases the level of P-VEGF only temporarily which may be associated with tumor ischemia. P-VEGF may be useful in predicting treatment response, monitoring disease course after TACE and judging the effect of different TACE regimens.
- Citation: Li X, Feng GS, Zheng CS, Zhuo CK, Liu X. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol 2004; 10(19): 2878-2882
- URL: https://www.wjgnet.com/1007-9327/full/v10/i19/2878.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i19.2878
Transcatheter arterial chemoembolization (TACE) is the most frequently applied palliative treatment in HCC patients who are considered to be unsuitable candidates for surgery. But the indications and outcome of TACE are not clearly defined in the literature[1,2]. TACE therapy is invasive and associated with high financial costs[3]. To predict which patients are likely to benefit from TACE would be useful to both oncologists and patients.
Tumor angiogenesis is essential for solid tumorigenesis, growth, invasion and metastasis[4-6]. Tumor angiogenesis is mediated by angiogenic factors and vascular endothelial growth factor (VEGF) is one of the most potent factors. Strong VEGF expression has been demonstrated in various solid tumor types. In HCC, VEGF expression in tumor tissue has been reported to be correlated with aggressive behavior, early metastasis spread, and poor prognosis[7,8]. Recently, the expression of VEGF in patients with various malignancies has been made possible by measuring circulating VEGF concentrations with the enzyme-linked immunosorbent assay (ELISA)[9]. The elevation of VEGF in blood implies a promotion of tumor angiogenesis, and several studies have revealed the predictive value of circulating VEGF level in disease progression and prognosis in various types of cancer[10,11]. Raised pre-treatment circulation VEGF levels in small-cell lung carcinoma, esophageal carcinoma, gastrointestinal cancer and non-Hodgkin’s lymphoma have been associated with a poor outcome[12-15].
Although elevated circulation VEGF levels have been measured in patients with various tumors. To our knowledge few data are available with regard to plasma VEGF (P-VEGF) levels in patients with HCC[16]. Data concerning the change of P-VEGF in the course of TACE therapy and its significance in prediction of patient’s response to chemoembolization have not been reported to date.
Therefore, in this prospective study, we analyzed the P-VEGF level in patients with HCC prior to treatment to determine the clinicopathologic significance of P-VEGF, and to assess the clinical usefulness of pre-treatment P-VEGF as a predictor of outcome in patients undergoing TACE therapy for HCC.
The study consisted of 45 patients (37 males, 8 females, mean age 50 years, range 29-77 years) with unresectable HCC who underwent TACE at the Department of Interventional Radiology, Wuhan Union Hospital between October 2000 and February 2002. HCC was diagnosed by distinctive radiographic findings, serum alpha fetoprotein, and with (n = 8) or without histological proof. All patients were treated with TACE therapy alone without any other therapies such as surgery, percutaneous ethanol injection, or systemic chemotherapy. The chemoembolization agents consisted of 100 mg/m2 of carboplatin, 50 mg/m2 of doxorubicin hydrochloride, 1 g of 5-fluorouracil and 5-25 mL of iodized oil (Lipiodol; Guerbet, Aulnay-sous-Bois, France). For those with hepatic arterial-portal vein fistula, gelfoam piece was used to block the fistula before iodized oil was used.
Twenty patients underwent TACE therapy for benign diseases (ten patients underwent partial hepatic artery embolization for hepatic cavernous angioma, ten patients underwent partial spleen artery embolization for hypersplenism) and seventeen healthy volunteers were also involved in this study, served as control to evaluate the P-VEGF cut-off level.
Physical examination, chest X-ray, routine liver function test were performed. Complete blood count, hepatitis B surface antigen status and serum alpha fetoprotein (α-FP) were prospectively collected from each patient. Tumor characteristics such as size, location, capsular, portal vein involvement, lymph node or extrahepatic metastasis, were assessed on the basis of radiographic findings. Tumor vascularity and arteio-portal vein shunting (A-V shunting) were studied by superselective digital subtraction angiography (DSA). Tumor staging was performed using the International Union Against Cancer (UICC) TNM classification[17].
Peripheral venous blood was taken before (n = 45) and 1 (n = 30), 3 (n = 44), 7 (n = 18) and 1 mo (n = 45) after TACE therapy. Blood samples were collected in sterile glass tubes containing 0.057 mL of 15% EDTA. Plasma was separated by centrifugation at 2000 r/min for 15 min to eliminate platelets and stored at -70 °C till use.
Plasma VEGF was measured in duplicate by commercially available ELISA kits (Quantikine human VEGF, R&D System, Minneapolis, MN, USA). According to the manufacturer the minimal detectable dose of VEGF was < 5.0 ng/L, the intra-assay and the inter-assay variabilities were 6.7%-5.1% and 8.8%-6.2%, respectively. Absorbance was determined using a biokinetics reader EL312e (Bio-Tek Instruments, Winooski, VT, USA) at 450 nm.
Response to treatment was assessed by image examinations one month after TACE Therapy. Treatment was evaluated according to the tumor volume change and retention rate of the iodized oil. The tumor volume was calculated using the summed products of the longest dimensions of the lesion, and patients were categorized as responding group (tumor size decrease or stable, no new-born nest) and non-responding group (enlargement of the tumor or the presents of new-born nest). According to the retention rate of the oil, patients were categorized as groups > 75%, 50%-75%, 25%-50% and < 25%.
Statistical analysis was carried out by the SPSS 10.0 packed program. To investigate the relationships between P-VEGF levels and other variables, t test, ANOVA test, Chi-square test, Fisher exact probability test, correlation coefficient (r) and spearman’s rank correlation (rs) were used when appropriate. P < 0.05 based on a two-tailed test was considered statistically significant.
The pre-operative P-VEGF levels were significantly higher in HCC patients (63.83 ± 51.09 ng/L) than those in benign liver diseases (28.99 ± 6.67 ng/L) ( P = 0.006) and in healthy controls (25.60 ± 5.47 ng/L) ( P = 0.003). There was no significant difference in P-VEGF levels between the patients with benign liver diseases and healthy control. The sensitivity of P-VEGF in detecting HCC, using the mean P-VEGF of the patients with benign liver diseases (28.99 ng/L) as cut-off level, was found to be 73.3% with 62.2% specificity.
There was no significant association between pre-TACE P-VEGF levels and various clinical and laboratory variables, except the gender (t = 4.111, P = 0.003). There was a weak correlation between P-VEGF levels and the platelet number (PLT) (r = 0.312, P = 0.038) and no correlation with the levels of α-FP (r = 0.068).
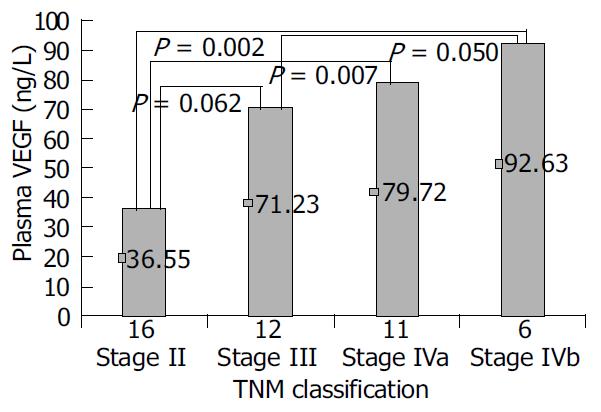
There was no significant association between the P-VEGF level and the presence of cirrhosis, tumor location, tumor capsule, ascites, lymph node metastasis, or the tumor vascularity. Significant differences were observed when P-VEGF was categorized by tumor size (P = 0.006), distant metastasis (P = 0.017), portal vein thrombosis (P = 0.011), arterio-portal vein shunting (P = 0.026), and advanced TNM stage (P = 0.044) (Table 1, Figure 1).
| Group | No. of patients | Median plasma VEGF (ng/L) | Statistic value | P value | |
| Cirrhotic liver | No | 13 | 80.15 | t = 1.380 | 0.175 |
| Yes | 32 | 57.19 | |||
| Tumor location | Left lobe | 4 | 77.82 | F = 0.275 | 0.843 |
| Right lobe | 26 | 66.62 | |||
| Spiegel’s lobe | 5 | 50.38 | |||
| Multiple lobe | 10 | 57.68 | |||
| Tumor capsule | No | 32 | 68.15 | t = 0.888 | 0.379 |
| Yes | 13 | 53.18 | |||
| Ascites | No | 37 | 62.81 | t = 1.151 | 0.280 |
| Yes | 8 | 82.60 | |||
| Lymph node involvement | No | 39 | 61.68 | t = 1.677 | 0.101 |
| Yes | 6 | 101.31 | |||
| Tumor vascularity | Hypervascularity | 31 | 75.59 | F = 0.922 | 0.406 |
| Median | 9 | 55.31 | |||
| Hypovascularity | 3 | 39.1 | |||
| Tumor size | < 5 cm | 7 | 27.57 | F = 5.804 | 0.006 |
| 5-10 cm | 22 | 55.59 | |||
| > 10 cm | 11 | 101.54 | |||
| Portal vein thrombosis | No | 37 | 57.97 | t = 2.669 | 0.011 |
| Yes | 8 | 104.79 | |||
| Extra hepatic metastasis | No | 39 | 59.39 | t = 2.483 | 0.017 |
| Yes | 6 | 116.18 | |||
| TNM classification | Stage II | 16 | 36.55 | F = 2.937 | 0.044 |
| Stage III | 12 | 71.23 | |||
| Stage IVa | 11 | 79.72 | |||
| Stage IVb | 6 | 92.63 | |||
| Arterio-portal vein shunting | No | 37 | 56.03 | t = 2.283 | 0.026 |
| Yes | 8 | 99.9 |
Using mean concentration of T II tumors as cut-off level, P-VEGF was able to predict T III/T IV tumors with 75.9% sensitivity and 62.5% specificity. Using mean concentration of portal vein thrombosis of negative tumors as cut-off level, P-VEGF was found to have a sensitivity of 62.5% and a specificity of 64.9% in detecting portal vein thrombosis of positive tumors. Using mean concentration of the distant metastasis-free group as cut-off level, P-VEGF was able to exclude distant metastasis with a negative predictive value of 89.3%, a sensitivity of 50% and a specificity of 64.1%. P-VEGF could predict the presence of A-V shunting with 75% sensitivity and 67.6% specificity by using mean concentration of the non-A-V shunting patients as cut-off level (Table 2).
| Cut-off VEGF | Sensitivity | Specificity | Positive | Negative predictive | |
| value (ng/L) | (%) | (%) | predictive value (%) | value (%) | |
| T II and T III/IV | 36.55 | 75.9 | 62.5 | 78.6 | 58.8 |
| Portal vein thrombosis | 57.97 | 62.5 | 64.9 | 27.8 | 88.8 |
| Extra hepatic metastasis | 59.39 | 50 | 64.1 | 17.6 | 89.3 |
| Arterio-portal vein shunting | 56.03 | 75 | 67.6 | 33.3 | 92.6 |
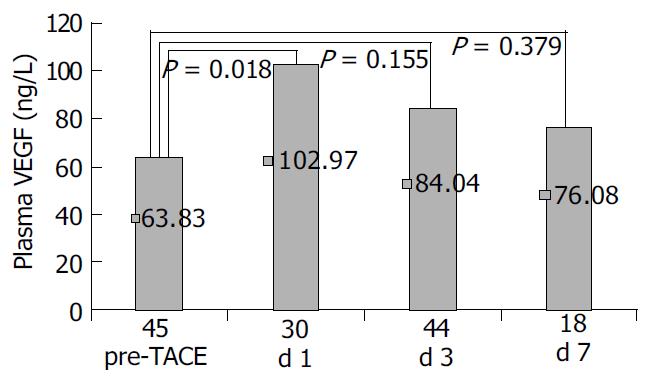
P-VEGF levels were significantly elevated in patients with HCC on the first post-TACE day (102.97 ± 89.26 ng/L) compared to pre-TACE (63.83 ± 51.09) ( P = 0.018). Then VEGF level decreased gradually to 84.04 ± 79.16 ng/L on the third day and 76.08 ± 45.38 ng/L on the seventh day post-TACE, showing no statistical difference with pre-TACE P-VEGF levels (Figure 2). A similar change of P-VEGF was found in patients who underwent TACE for treatment of benign liver diseases (data not shown).
One month after TACE, the P-VEGF level was 71.47 ± 50.87 ng/L, no statistical difference was found between them and pre-TACE P-VEGF levels.
Treatment response was evaluated according to the change in tumor volume and retention of the oil. Forty five patients were categorized as responding group (18 cases) and non-responding group (27 cases). The change rate of P-VEGF levels was associated with the change in tumor volume (rs = 0.340, P = 0.034). No statistic difference was found between changes in α-FP levels and tumor volume (rs = 0.230, P = 0.138).
The pre-TACE P-VEGF level in non-responding group (73.33 ± 70.32 ng/L) was significantly higher than that in the responding group (49.52 ± 25.33 ng/L, P = 0.025). The response rate of the low VEGF group (P-VEGF < 49.5 ng/L) was significantly higher than that of the high VEGF group (P-VEGF > 49.5 ng/L) (51.6% vs 14.29%; P = 0.018, Fisher exact probability test) (Table 3).
| Clinical response | Plasma VEGF (ng/L) | No. of patients | |||
| Low VEGF | High VEGF | ||||
| Responder | 49.52 ± 25.33 | t = 2.285 | 16 | 2 | χ2 = 5.599 |
| Non-responder | 73.33 ± 70.32 | P = 0.025 | 15 | 12 | P = 0.018 |
According to the retention rate of the iodized oil, 45 patients were classified as follows: 11 cases > 75%, 16 cases 50%-75%, 9 cases 25%-50% and 9 cases < 25%. The change rate of P-VEGF levels was strongly associated with oil retention (rs = 0.494, P = 0.001). No statistical difference was found between the change rate of α-FP and oil retention (rs = 0.209, P = 0.179).
By alternate splicing of the VEGF gene, VEGF could exist at least in one of the four forms, namely, VEGF206 (tissue bound), VEGF189 (tissue bound), VEGF165 (the most abundant form, soluble, and partly tissue bound) and VEGF121 (soluble)[18]. Compared with bioassays, detection of soluble VEGF isoforms with immunoassay was characterized by low detection limits and greater specificity, reproducibility, and practicability[19,20].
Recently, serum VEGF was measured by an enzyme-linked immunosorbent assay (ELISA) in patients with various types of cancer[20]. Increased levels of VEGF were demonstrated in most patients with cancer and closely related to tumor progression and metastasis[21,22]. However, some studies have shown that VEGF is not only secreted from tumor cells but also from a variety of CD34+ hematological cells (including platelets, megakaryocytes, monocytes, T-lymphocytes and leukocytes) during clotting[23]. Serum VEGF concentrations could reflect blood platelet counts rather than VEGF synthesis by tumor tissues, and plasma rather than serum should be considered suitable for the measurement of circulating VEGF[24]. So in our study, plasma VEGF concentrations were analyzed and ethylenediamine tetraacetic acid (EDTA) was used as anticoagulant.
Hepatocellular carcinoma (HCC) is a highly vascular tumor characterized by neovascularization and a high propensity for venous invasion. A strong VEGF expression was observed in the tissue of HCC and closely associated with tumor progression and metastasis[6]. Recently, Poon et al[25] reported that serum VEGF level was significantly elevated in patients with HCC, and significantly high serum VEGF levels were associated with the absence of tumor capsule, the presence of venous invasion and microsatellite modules, and advanced TNM stage. Li et al[26] also considered that serum VEGF was a predictor of invasion and metastasis of HCC. To our knowledge, only one previous study measured P-VEGF changes in HCC patients. Jinno et al[16] reported that P-VEGF was a promising tumor marker for remote metastasis of HCC and its specificity, sensitivity and overall accuracy were high enough to be clinically useful.
In the current study, pre-TACE P-VEGF was found to be markedly elevated in the majority of patients with HCC and the increase was closely related to a more advanced stage of diseases. This finding suggested that HCC cells were an important source of P-VEGF. But the lower P-VEGF levels in HCC patients overlapped considerably with those in normal controls, thus limiting the application of VEGF as a tumor marker in early detection of HCC.
We found that high P-VEGF levels were associated with the presence of extrahepatic metastasis and portal vein involvement. Because vascular invasion by tumor cells is indispensable for remote metastasis, metastasis, portal vein involvement and A-V shunting can reflect the ability of tumor cells to invade blood vessels. These results indicated that P-VEGF could be used as a tumor marker in detecting vascular invasive phenotypes of HCC. It is clinically important to search for the metastasis radiologically in patients with high P-VEGF levels.
In the present study, there was a weak correlation of PLT number with P-VEGF level. It has been suggested that platelets aggregate at metastatic sites, due to factors released from metastatic cells and vascular invasion, resulting in microthrombosis, tumor adhesion, and may release VEGF to the circulation[27,28]. Therefore, we considered that in metastatic HCC patient platelet activation was a part of the sources of plasma VEGF.
The finding that there was no significant association between the P-VEGF level and tumor vascularity shown by DSA is of great interest. This might suggest that circulation VEGF levels could reflect the formation of new vessels rather than the degree of existing tumor vascularity.
No correlation was found between P-VEGF and serum α-FP, suggesting that they had different mechanisms of production, and P-VEGF might be an independent predictive factor.
Suzuki et al[29] found serum VEGF increased significantly and reached the peak value 7 d after TACE, suggesting that VEGF might play a role as a sensitive marker for tumor ischemia. We observed that P-VEGF increased and reached the peak value on the first day after TACE and then decreased gradually. We thought that the changes of serum VEGF during TACE might be associated with chemotherapy-induced thrombocytopenia and a subsequent rebound increase in platelet number[30]. Although the exact mechanism is currently unknown, hypoxia caused by TACE, clotting and platelets aggregation after TACE, tumor cell lysis (and release of VEGF)[31], elevation of VEGF in acute hepatitis[32], and tissue trauma[33] might contribute to the increase of P-VEGF in the early post-TACE period.
It has been reported that high pre-treatment levels of circulating VEGF are correlated with a poor response to the conventional chemotherapy[12-15]. Similar results were found that the mean P-VEGF of the responding group was significantly higher than that of the non-responding group. Two treatment response indicators (tumor volume change and retention of the iodized oil) were associated with the change rate of P-VEGF levels rather than the change rate of α-FP. These results indicated that P-VEGF might act as a follow-up marker for post-TACE monitoring of the disease course and evaluation of curative effect, especially in α-FP negative patients. It may be useful in predicting treatment response and judging the effect of different TACE regimens.
In summary, P-VEGF level is an independent predictive factor of tumor progression, especially vascular invasion. TACE increases the level of P-VEGF only temporarily which may be associated with tumor ischemia. P-VEGF may be useful in predicting treatment response, monitoring disease course after TACE and judging the effect of different TACE regimens.
Edited by Kumar M and Wang XL Proofread by Xu FM
| 1. | Okada S. Transcatheter arterial embolization for advanced hepatocellular carcinoma: the controversy continues. Hepatology. 1998;27:1743-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Llad Inverted Question Marko L, Virgili J, Figueras J, Valls C, Dominguez J, Rafecas A, Torras J, Fabregat J, Guardiola J, Jaurrieta E. A prognostic index of the survival of patients with unresectable hepatocellular carcinoma after transcatheter arte-rial chemoembolization. Cancer. 2000;88:50-57. [RCA] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 3. | Katyal S, Oliver JH, Peterson MS, Chang PJ, Baron RL, Carr BI. Prognostic significance of arterial phase CT for prediction of response to transcatheter arterial chemoembolization in unresectable hepatocellular carcinoma: a retrospective analysis. AJR Am J Roentgenol. 2000;175:1665-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4848] [Cited by in RCA: 4796] [Article Influence: 165.4] [Reference Citation Analysis (0)] |
| 5. | Kirsch M, Schackert G, Black PM. Metastasis and angiogenesis. Cancer Treat Res. 2004;117:285-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Gupta MK, Qin RY. Mechanism and its regulation of tumor-induced angiogenesis. World J Gastroenterol. 2003;9:1144-1155. [PubMed] |
| 7. | Yamaguchi R, Yano H, Iemura A, Ogasawara S, Haramaki M, Kojiro M. Expression of vascular endothelial growth factor in human hepatocellular carcinoma. Hepatology. 1998;28:68-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 270] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Poon RT, Lau CP, Ho JW, Yu WC, Fan ST, Wong J. Tissue factor expression correlates with tumor angiogenesis and invasiveness in human hepatocellular carcinoma. Clin Cancer Res. 2003;9:5339-5345. [PubMed] |
| 9. | Kondo S, Asano M, Matsuo K, Ohmori I, Suzuki H. Vascular endothelial growth factor/vascular permeability factor is detectable in the sera of tumor-bearing mice and cancer patients. Biochim Biophys Acta. 1994;1221:211-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 135] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Kraft A, Weindel K, Ochs A, Marth C, Zmija J, Schumacher P, Unger C, Marmé D, Gastl G. Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer. 1999;85:178-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 11. | Fuhrmann-Benzakein E, Ma MN, Rubbia-Brandt L, Mentha G, Ruefenacht D, Sappino AP, Pepper MS. Elevated levels of angiogenic cytokines in the plasma of cancer patients. Int J Cancer. 2000;85:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Salven P, Teerenhovi L, Joensuu H. A high pretreatment serum vascular endothelial growth factor concentration is associated with poor outcome in non-Hodgkin's lymphoma. Blood. 1997;90:3167-3172. [PubMed] |
| 13. | Salven P, Ruotsalainen T, Mattson K, Joensuu H. High pre-treatment serum level of vascular endothelial growth factor (VEGF) is associated with poor outcome in small-cell lung cancer. Int J Cancer. 1998;79:144-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Shimada H, Takeda A, Nabeya Y, Okazumi SI, Matsubara H, Funami Y, Hayashi H, Gunji Y, Kobayashi S, Suzuki T. Clinical significance of serum vascular endothelial growth factor in esophageal squamous cell carcinoma. Cancer. 2001;92:663-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Hyodo I, Doi T, Endo H, Hosokawa Y, Nishikawa Y, Tanimizu M, Jinno K, Kotani Y. Clinical significance of plasma vascular endothelial growth factor in gastrointestinal cancer. Eur J Cancer. 1998;34:2041-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 136] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Jinno K, Tanimizu M, Hyodo I, Nishikawa Y, Hosokawa Y, Doi T, Endo H, Yamashita T, Okada Y. Circulating vascular endothelial growth factor (VEGF) is a possible tumor marker for metastasis in human hepatocellular carcinoma. J Gastroenterol. 1998;33:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 87] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Zhang Z, Wu M, Shen F. [Significance of TNM clasification in prognostic evaluation of hepatocelluar carcinoma following surgical resection]. Zhonghua ZhongLiu ZaZhi. 1999;21:293-295. [PubMed] |
| 18. | Houck KA, Ferrara N, Winer J, Cachianes G, Li B, Leung DW. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991;5:1806-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 894] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 19. | Bienvenu JA, Monneret G, Gutowski MC, Fabien N. Cytokine assays in human sera and tissues. Toxicology. 1998;129:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Yamamoto Y, Toi M, Kondo S, Matsumoto T, Suzuki H, Kitamura M, Tsuruta K, Taniguchi T, Okamoto A, Mori T. Concentrations of vascular endothelial growth factor in the sera of normal controls and cancer patients. Clin Cancer Res. 1996;2:821-826. [PubMed] |
| 21. | Zhao J, Hu J, Cai J, Yang X, Yang Z. Vascular endothelial growth factor expression in serum of patients with hepatocellular carcinoma. Chin Med J (Engl). 2003;116:772-776. [PubMed] |
| 22. | Dirix LY, Vermeulen PB, Pawinski A, Prové A, Benoy I, De Pooter C, Martin M, Van Oosterom AT. Elevated levels of the angiogenic cytokines basic fibroblast growth factor and vascular endothelial growth factor in sera of cancer patients. Br J Cancer. 1997;76:238-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 202] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Lee JK, Hong YJ, Han CJ, Hwang DY, Hong SI. Clinical usefulness of serum and plasma vascular endothelial growth factor in cancer patients: which is the optimal specimen? Int J Oncol. 2000;17:149-152. [PubMed] |
| 24. | Jelkmann W. Pitfalls in the measurement of circulating vascular endothelial growth factor. Clin Chem. 2001;47:617-623. [PubMed] |
| 25. | Poon RT, Ng IO, Lau C, Zhu LX, Yu WC, Lo CM, Fan ST, Wong J. Serum vascular endothelial growth factor predicts venous invasion in hepatocellular carcinoma: a prospective study. Ann Surg. 2001;233:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 185] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 26. | Li XM, Tang ZY, Qin LX, Zhou J, Sun HC. Serum vascular endothelial growth factor is a predictor of invasion and metastasis in hepatocellular carcinoma. J Exp Clin Cancer Res. 1999;18:511-517. [PubMed] |
| 27. | Pinedo HM, Verheul HM, D'Amato RJ, Folkman J. Involvement of platelets in tumour angiogenesis? Lancet. 1998;352:1775-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 260] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 28. | Möhle R, Green D, Moore MA, Nachman RL, Rafii S. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci USA. 1997;94:663-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 524] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 29. | Suzuki H, Mori M, Kawaguchi C, Adachi M, Miura S, Ishii H. Serum vascular endothelial growth factor in the course of transcatheter arterial embolization of hepatocellular carcinoma. Int J Oncol. 1999;14:1087-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Verheul HM, Hoekman K, Luykx-de Bakker S, Eekman CA, Folman CC, Broxterman HJ, Pinedo HM. Platelet: transporter of vascular endothelial growth factor. Clin Cancer Res. 1997;3:2187-2190. [PubMed] |
| 31. | Nasu R, Kimura H, Akagi K, Murata T, Tanaka Y. Blood flow influences vascular growth during tumour angiogenesis. Br J Cancer. 1999;79:780-786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Akiyoshi F, Sata M, Suzuki H, Uchimura Y, Mitsuyama K, Matsuo K, Tanikawa K. Serum vascular endothelial growth factor levels in various liver diseases. Dig Dis Sci. 1998;43:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Bondestam J, Salven P, Jääskela-Saari H, Ikonen T, Lepäntalo M, Mattila S, Joensuu H. Major surgery increases serum levels of vascular endothelial growth factor only temporarily. Am J Surg. 2000;179:57-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |