Published online Oct 1, 2004. doi: 10.3748/wjg.v10.i19.2859
Revised: April 22, 2004
Accepted: April 29, 2004
Published online: October 1, 2004
AIM: Pelvic magnetic resonance imaging (MRI) and endoanal ultrasound which are established imaging methods for perianal inflammatory lesions in patients with Crohn’s disease require expensive specialized equipments and expertise. We investigated the feasibility and sensitivity of transcutaneous perianal ultrasound (PAUS) using regular ultrasound probes in the imaging of perianal inflammatory lesions. The sonographic findings were correlated to pelvic MRI-scans.
METHODS: We performed PAUS in 25 patients with Crohn’s disease and clinical signs of perianal inflammatory disease. Within a median of 10 d (range 0-75) these patients underwent MRI of the pelvis. Regular convex and linear high resolution probes were used for PAUS. The sonographic findings were correlated to the MRI findings by blinded investigators.
RESULTS: The sonographic investigations were well tolerated by all patients. Fistulae typically presented as hypoechoic tracks. Twenty-nine fistulae were detected in 22 patients. Abscesses were detected in 7 patients and presented as hypo- or anechoic formations. Twenty-six of 29 fistulae and 6 of 7 abscesses could be confirmed by MRI. Kappa statistics showed an excellent agreement (kappa > 0.83) between the two imaging methods.
CONCLUSION: PAUS is a simple, painless, feasible, real-time method that can be performed without specific patient preparation which is comparable in its sensitivity to pelvic MRI in the detection of perianal fistulae and/or abscesses. PAUS can especially be recommended as a screening tool in acute perianal disorders such as perianal abscess and for follow-up studies of perianal inflammatory disease.
- Citation: Wedemeyer J, Kirchhoff T, Sellge G, Bachmann O, Lotz J, Galanski M, Manns MP, Gebel MJ, Bleck JS. Transcutaneous perianal sonography: A sensitive method for the detection of perianal inflammatory lesions in Crohn’s disease. World J Gastroenterol 2004; 10(19): 2859-2863
- URL: https://www.wjgnet.com/1007-9327/full/v10/i19/2859.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i19.2859
Perianal disease is a common complication in Crohn’s disease. Approximately 20% of patients with Crohn’s disease would suffer from perianal fistulae and/or sinus tracks[1,2]. The diagnosis and treatment of perianal fistulae can be difficult and represent a challenge for physicians. Treatment modalities include conservative as well as surgical approaches. Therefore comprehensive imaging is necessary in order to develop adequate treatment strategies and to evaluate treatment outcomes[3]. Endoanal ultrasound (EAUS) as well as MRI of the pelvis are frequently used for imaging of perianal fistulae, sinus tracks or abscesses. The combination of MRI and EAUS is capable of detecting perianal fistulae with a sensitivity of 100%[4,5]. Recent studies for the medical treatment of perianal disease used either MRI of the pelvis or transanal ultrasound as a follow-up tool to evaluate the therapy outcomes after infliximab treatment[6-8].
However, both imaging methods require specialized and fairly expensive equipments as well as an experienced investigator. The use of a rigid EAUS probe can be painful or even impossible in patients with inflammatory perianal disease due to anal stenosis. Furthermore, EAUS does not allow the assessment of pathological changes extending to the gluteal region. MRI allows the evaluation of the whole pelvic region, however, it lacks the real time capacity of ultrasound and the high resolution of high frequency linear ultrasound probes.
Transcutaneous perineal/perianal ultrasound (PAUS) represents another method to detect perianal inflammatory disease which can be performed using regular ultrasound probes without special patient preparation. Furthermore, it can be used in patients with anal stenosis. However, despite its methodical simplicity, PAUS is not yet widely used in the detection of perianal fistulae and/or abscesses. Several investigators have applied perineal ultrasound in female patients for diagnosis of incontinence including postpartum anal sphincter tears[9-15]. These investigations were regularly performed in the lithotomy position and supplemented or compared with transvaginal or transrectal ultrasound. Rubens et al[16] described PAUS as a valuable tool to image perianal inflammatory disease in their pictorial essay, while Stewart et al[17] recently described PAUS using a combination of transvaginal and transperineal ultrasound in female and transperineal ultrasound in male patients to detect perianal fistulas and abscesses. Beside a linear probe, Parks et al[18] used a transvaginal probe for the PAUS to take advantage of its small footprints and they were able to classify fistulae according to the Parks classification. In their recent published study Mallouhi et al[19] showed a high correlation of perineal ultrasound findings and surgical examination. However, as Mallouhi et al[19] pointed out correctly surgical exploration in some cases, might not allow the detection of every perianal fistula. Despite the obvious advantages of perianal/perineal ultrasound no studies exist so far that compared the effectiveness of PAUS with the established imaging methods such as MRI and/or EAUS. Therefore, we investigated patients with clinical signs of perianal fistulae or abscesses using PAUS and compared the detected pathology with results of MRI scans of the pelvis as the gold standard. Also, we performed follow-up studies in certain patients to monitor the treatment effectiveness.
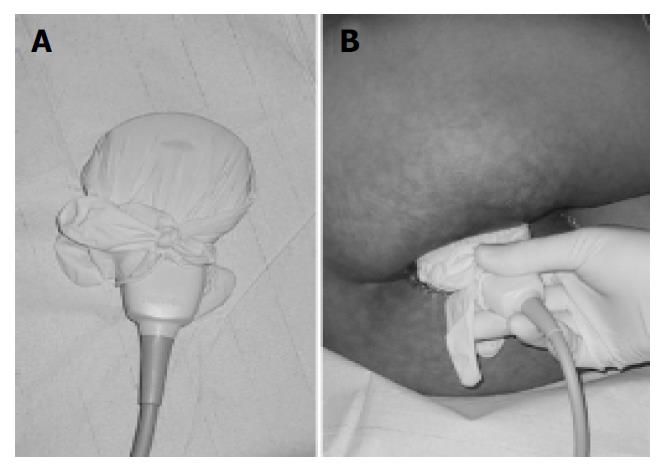
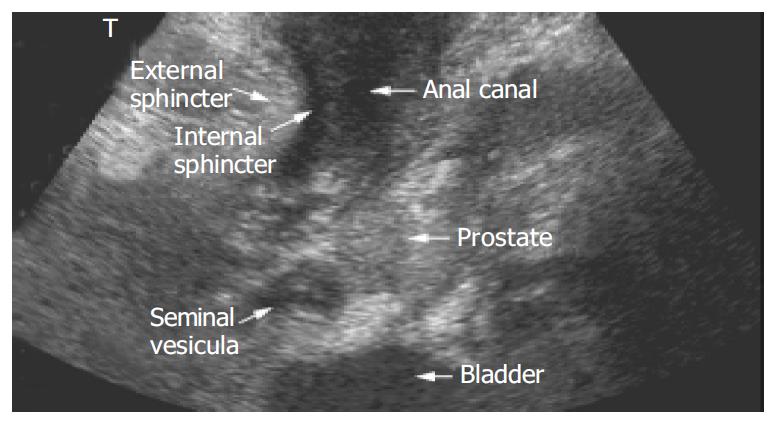
Patients were recruited either from the inflammatory bowel disease outpatient clinic or from the gastroenterology wards of our institution. Ultrasound examinations were performed in 25 patients (17 females, 8 males) with a mean age of 36.2 ± 2.5 years. All patients had prediagnosed Crohn’s disease confirmed by biopsy and presented with complaints suspicious of perianal disease. No specific preparations of the patients such as enemas were performed. Either a Toshiba Aplio (3.3 Mhz [Tissue Harmonic Imaging, THI] and 12 Mhz transducer), a Toshiba Powervision (6 Mhz and 12 Mhz transducer, Toshiba, Neuss, Germany) or a Siemens Elegra (3.75 [THI] and 9 Mhz transducer, Siemens, Erlangen, Germany) was used depending on availability for the ultrasound examination. Colour Doppler ultrasound was applicable with all probes. The ultrasound probe was covered with contact gel, and introduced into a regular latex examination glove with the fingers of the glove tied up in knots for hygienic reasons (Figure 1A). For sufficient contact, contact gel was spread on the outside of the glove before the examination was performed. PAUS was performed with the patient in the left lateral position by placing the probe directly above the anus in the sagittal plane (Figure 1B). For better orientation, the examination was started with a convex transducer identifying bladder, rectum and prostate/vagina (Figure 2). Details and pathological findings were imaged and further investigated using the linear high resolution probes. The maximal penetration for good visualisation using linear transducers was 6 cm. Depending on the specific findings the probe was turned or shifted in the perianal area. The probe was placed above any pathological opening. Colour Doppler was used to distinguish blood vessels from other anechoic/hypoechoic lesions such as fistulae. All ultrasound examinations were checked by either J.S.B. or M.J.G. who both held the highest DEGUM (German society for ultrasound in medicine) ultrasound certificates (DEGUM III, Seminarleiter). Colour Doppler ultrasound was applicable with all probes. Fistulae were catagorized as superficial, intersphincteric, transsphincteric suprasphincteric, extrasphincteric, recto-vaginal fistulae and/or abscesses.
Within a median of 10 d (range 0-75) the patients were also examined by pelvic MRI. MRI was performed to identify inflammatory changes, abscess formations and fistulae. A 1.5 Tesla MR scanner (GE Signa Horizon Echospeed, Milwaukee, Wisconsin) was used. A phased array surface coil was placed on the pelvis. Transverse and sagittal T1-spin echo (T1SE), fat-saturated T2-fast spin echo (fsT2FSE) sequences were applied. Also, transverse and oblique coronal (angulated adjacent to the anal canal) T2FSE sequences without fat saturation were applied in order to detect fistulous tracks. Either respiratory compensation or respiratory trigger was used to avoid artefacts from respiratory motion. After intravenous gadolinium administration (0.1 mmol/kg Gd-DTPA, Magnevist, Schering, Berlin, Germany) transverse and oblique coronal fat-saturated T1-spin echo (fsT1SEpostGd) sequences were performed. Raised signal intensity in the T2-weighted images and an increased uptake of Gd-DTPA served as markers for active inflammatory changes, whereas low signal intensity bands distorting normal fibrofatty tissue in both T1 and T2 weighted images were considered to be fibrous scarring. The results were reviewed by two radiologists in consensus reading. The investigators of either method were not aware of the results generated by the other.
Results were compared by calculating the kappa coefficient according to Cohen.
PAUS was well tolerated in all patients. In cases of subcutaneous perianal abscesses or significant inflammatory changes, slight local pain was reported during the examination when pressure was applied with the ultrasound probe. Two patients who had a history of significant anal stenosis and repeated surgical anal dilations were included in the study and evaluated with PAUS, because endoanal ultrasound could not be performed in these cases.
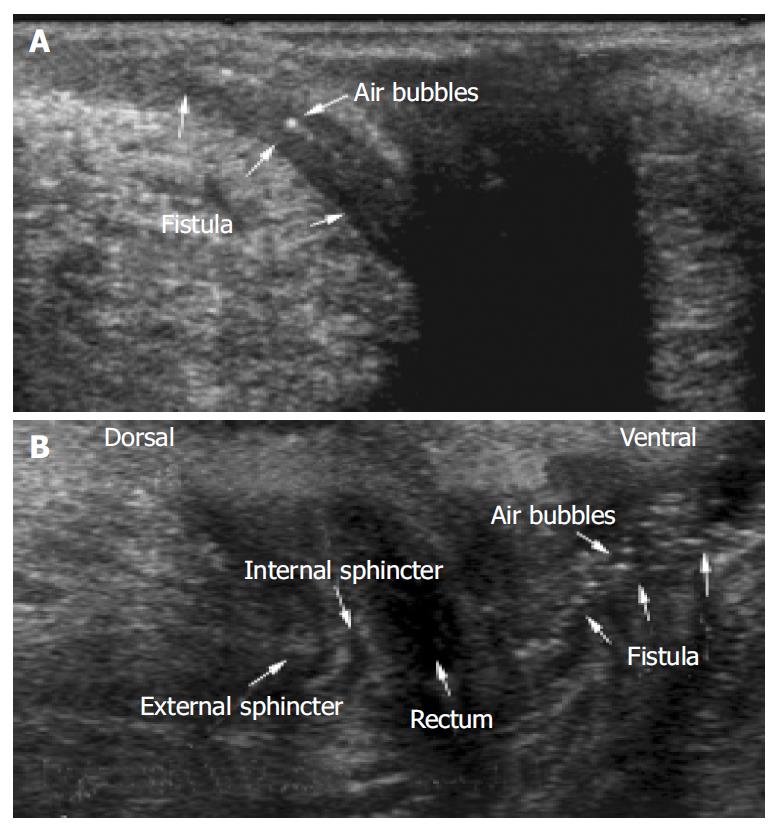
A general view was achieved using a regular convex probe (as listed in the Materials and Methods section) that showed rectum, bladder, prostate gland and urethra or vagina and uterus (Figure 2). Small intestinal bowel loops could be shown as well as pathological pelvic intraabdominal fluid collections. However, for comprehensive imaging of perianal inflammatory disease, a high frequency linear probe provided a much more detailed imaging. The penetration that could be achieved using a linear transducer (as listed in Materials and Methods) was sufficient to detect most of the perianal inflammatory changes. The anal canal presented as an anechoic area (Figure 3A, B). The external anal sphincter presented with hyperechoic striped bands while the internal sphincter had a homogenic hypoechoic texture (Figure 3B).
Pathological findings were detected in 96% (24/25) of the patients examined with PAUS. Pathological findings included entero-cutaneous perianal fistulae, sinus tracts without cutaneous openings, bifurcated complex perianal fistulae, perianal or subcutaneous abscesses, rectovaginal fistulae and inflammatory remnants post perianal abscess.
In 88% (22/25) of patients investigated, fistulae and/or sinus tracts could be visualized by PAUS. A total of 29 fistulae were detected. Fistulae and sinus tracks presented as hypo/anechoic tracks. Figure 3 shows typical PAUS images of entero-cutaneous perianal fistulae. Regularly small hyperechoic dots could be detected representing air bubbles within the fistulous tracks. In 45% (10/22) of the fistulae, it was possible to trace small hyperechoic dots which would move within the fistulae and therefore enabling the investigator to detect even minor fistulous tracks indicating active fistulae.
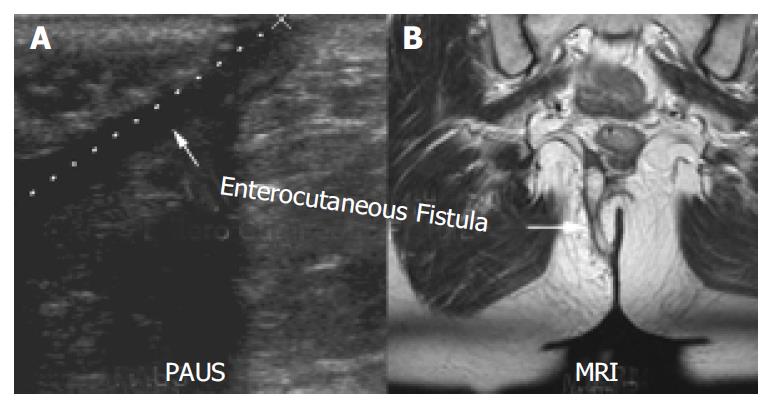
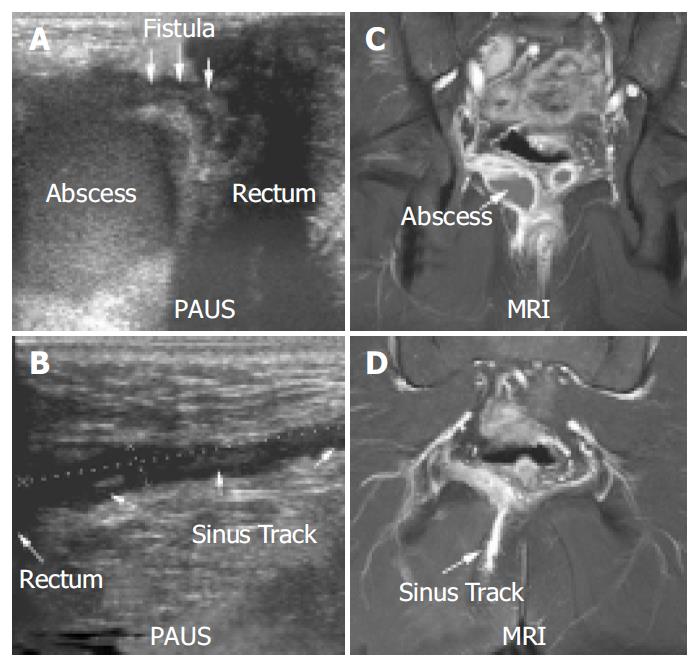
MRI confirmed 90% (26/29) fistulae in 91% (20/22) of these patients. All fistulae detected by MRI were also detected by PAUS. The kappa coefficient for fistulae was calculated as 0.83 resembling an excellent agreement. In two patients both methods described incomplete fistulae (sinus tracks with no visible cutaneous opening). Figure 4 shows a perianal enterocutaneous fistula imaged by PAUS and MRI.
As PAUS included inspection of the anoderm, cutaneous openings of fistulae could easily be detected. In two cases MRI was not able to confirm perianal fistulae that were detected by PAUS. However, discharge from a cutaneous opening proved the presence of a perianal fistula in both of these cases, which could be visualized with PAUS. Both of these fistulae were short intersphincteric fistulae of type 1 according to the Parks classification[18]. In one of these cases the fistula described in PAUS could be detected in a repeat MRI performed using a vaginal tampon for better anatomical differentiation. However, the investigators were aware of the findings made in the PAUS in this second examination.
Follow-up PAUS investigations were performed in 8 of the 25 patients. Improvement under therapy was noted in 4 cases, no change in 2 cases and progressive disease in 2 patients. The sonographical improvement was consistent with clinical findings. In one case follow-up MRI was performed confirming the PAUS findings as both imaging methods described an abscess within the fistulous track.
PAUS was able to visualize rectovaginal fistulae in 3 cases. MRI confirmed recto-vaginal fistulae in two of the three cases. In the remaining case MRI showed a fistula from the sigmoid colon to the vagina, in contrast, PAUS described a recto-vaginal fistula. While gynaecological examination did not confirm the presence of any fistula, drainage of blue stain into the vagina after the introduction of a methylene blue soaked swab into the rectum finally proved the presence of a recto-vaginal fistula as was described by PAUS. The sigmoid-vaginal fistula was not detected in the PAUS examination.
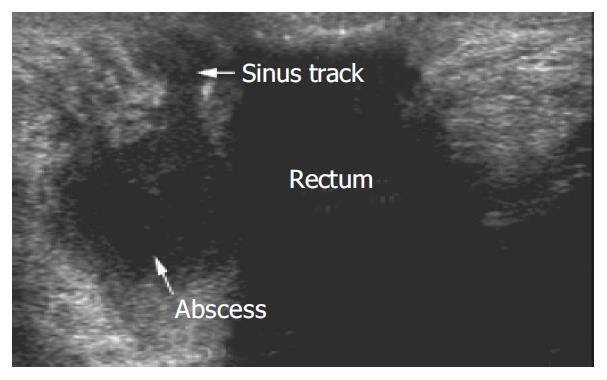
Perianal fluid collections/abscesses were detected by PAUS in 28% (7/25) of the patients. Abscesses presented as oval shaped hypo- to anechoic masses which were demarcated off the regular tissue by a hypoechoic seam (Figure 5). In 5 cases a direct association of a fistulous and/or sinus track to the abscess formation could be demonstrated. Figure 5 shows a perianal sonographic scan of a male patient that presented to our outpatient clinic with severe perianal discomfort. While inspection and digital examination of the anus were inconspicuous, the transcutaneous perianal ultrasound examination revealed hypoechoic fluid collections dorsal of the rectum in this patient. The abscess shown in Figure 5 had a fistulous track connecting the rectum and a complex intrasphincteric fistulous system. In 6 cases fluid collections could be confirmed using MRI, and no fluid collection detected in MRI was missed by PAUS. In one case MRI could not confirm a fluid collection. However the two examinations were performed at an interval of 23 d. The smallest abscess detected in PAUS measured 14 mm × 12 mm × 8 mm. In three of the cases with significant, drainable abscesses examination under anaesthesia (EUA) could confirm PAUS/MRI findings.
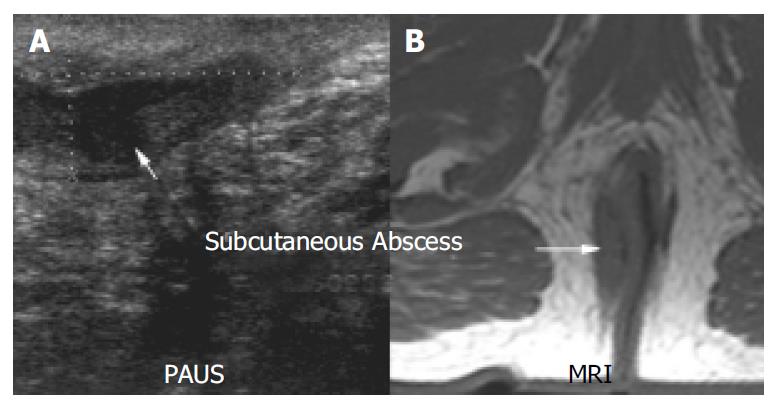
Figure 6 shows an example of a perianal abscess as imaged by PAUS and MRI. Clinical inspection of the anal region did not show any abnormalities in this patient. However anal discharge upon digital examination was observed and further imaging was initiated. Both PAUS and MRI showed a significant fluid collection with a diameter of approximately 4.5 cm. In contrast to MRI, PAUS detected a fistulous connection to the rectum. In addition to the abscess, a sinus track was detected that emanated from the abscess, and ended within the gluteal fat. The sinus tract could clearly be visualized by both methods (Figure 6). Interestingly, EUA failed to reproduce the pathological findings in the first place and had to be repeated to confirm the PAUS/MRI findings and to achieve appropriate drainage of the abscess. In Figure 7 a small subcutaneous abscess was imaged by PAUS and MRI. Both methods were able to show an active fistulous track reaching from the rectum to the subcutaneous fluid collection.
In this study we investigated the feasibility of transcutaneous PAUS for the evaluation of perianal inflammatory disorders in patients with Crohn´s disease.
We showed that perianal fistulae, complex fistulous systems as well as perianal abscesses could be detected with high sensitivity using PAUS. The results were comparable to pelvic MRI that currently represents in combination with EAUS, the gold standard for the imaging of perianal inflammatory disease. However, PAUS still has certain limitations when performed in the left lateral position compared to EAUS and MRI. The localization of fistulae within the sphincter apparatus is important when surgical interventions are indicated. While imaging of the internal anal sphincter is difficult using PAUS, the external sphincter can be clearly distinguished due to its hyperechoic striped appearance. In terms of impact on treatment, the definite description of the anatomical relationship of pathological changes to the external anal sphincter according to the Parks classification is most important[18]. Still, accurate localization of pathological changes to the internal/external sphincter requires an experienced investigator.
Despite the limitations stated above, we believe that PAUS has several advantages compared to EAUS and MRI. PAUS is as effective as MRI in the detection of perianal fistulae, but the detection of air bubbles within a fistulous track can clearly indicate active fistulae. Real time imaging in sonography is an advantage that can be helpful in detecting possible connection to the rectal lumen. In two cases investigated by MRI and PAUS, MRI was not able to detect a short perianal fistulae, while the cutaneous openings of the fistulae were obvious on clinical inspection. While EAUS is limited to pathological changes associated to the rectal lumen, perianal ultrasound can be used to identify fistulous tracks in the gluteal region. PAUS therefore combines the high resolution and real time capabilities of EAUS and the panoramic view generated by MRI.
The presence of perianal abscesses is an important condition for surgical interventions in patients with perianal Crohn´s disease. Abscesses were detected by PAUS with the same sensitivity as by MRI. PAUS therefore is a valuable tool in the screening for perianal disease. Especially when immediate action is necessary and sophisticated diagnostic imaging could delay diagnosis due to limited availability, PAUS might be a feasible alternative to EAUS and MRI. For example, we were able to detect perianal abscesses in two patients that presented with severe perianal discomfort using PAUS. These patients did not have a history of perianal inflammatory disease and neither inspection of the anus nor digital rectal examination revealed pathological findings. These two patients underwent immediate surgical drainage of their abscesses without further imaging and therefore had to be excluded from this study. Vice versa a perianal abscess was suspected in a patient that presented with a painful perianal swelling with typical redness and induration. An abscess was ruled out using PAUS and the patient was spared a surgical intervention and had complete remission of symptoms. Again no further imaging was performed in this patient. In the future, a mobile application of PAUS in the operating room to guide drainage operations is conceivable. This, however, has not been tested in this study yet.
With more advanced imaging techniques in medicine, the costs of diagnosis and treatment have become a serious and important issue. Especially in chronic diseases, such as Crohn´s disease which require regular examination for follow-up and treatment-evaluation alternative, easily available methods to EAUS and MRI as rather expensive diagnostic tools are more than welcome. We believe that PAUS represents such an alternative for it can be performed with regular ultrasound probes and is therefore widely available. It is fast and does not require special patient preparations such as enemas or injection of contrast agents. Basically it can be performed in any outpatient radiology or internal medicine practice. Depending on on-site experience additional imaging of perianal disease with EAUS or MRI might be necessary. However, after initial comparison with the established methods, follow-up can easily be performed with PAUS alone. In follow-up studies the application of more sophisticated imaging methods such as EAUS or MRI would then be limited to investigate significant clinical changes not detectable by the transcutaneous approach.
In general PAUS was well tolerated by our patients. As a matter of fact one of the reasons to perform this study was that several patients refused diagnosis with EAUS. As perianal inflammatory disease can result in fibrosis and consecutive anal stenosis, EAUS might not be applicable at all in some patients.
The use of contrast agents such as hydrogen peroxide or Levovist might improve the imaging quality of perianal ultrasound[20-23]. However, the main goal of this study was to determine whether a simple alternative approach was helpful in the imaging of perianal disorders.
We would like to encourage the readers of this article who perform EAUS to employ PAUS using regular ultrasound probes. Since PAUS has been established in our department, the use of EAUS for inflammatory disease has been drastically reduced and is now limited to very few cases that can not be clarified with PAUS and/or MRI.
In conclusion, PAUS represents a sensitive, non-invasive tool that can be used for initial screening and follow-up in the detection of perianal inflammatory disease.
Edited by Zhang JZ and Wang XL Proofread by Xu FM
| 1. | Hellers G, Bergstrand O, Ewerth S, Holmström B. Occurrence and outcome after primary treatment of anal fistulae in Crohn's disease. Gut. 1980;21:525-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 323] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 2. | Schwartz DA, Loftus EV, Tremaine WJ, Panaccione R, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of fistulizing Crohn's disease in Olmsted County, Minnesota. Gastroenterology. 2002;122:875-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 706] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 3. | Sandborn WJ, Fazio VW, Feagan BG, Hanauer SB. AGA technical review on perianal Crohn's disease. Gastroenterology. 2003;125:1508-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 408] [Article Influence: 18.5] [Reference Citation Analysis (1)] |
| 4. | Orsoni P, Barthet M, Portier F, Panuel M, Desjeux A, Grimaud JC. Prospective comparison of endosonography, magnetic resonance imaging and surgical findings in anorectal fistula and abscess complicating Crohn's disease. Br J Surg. 1999;86:360-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 126] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Schwartz DA, Wiersema MJ, Dudiak KM, Fletcher JG, Clain JE, Tremaine WJ, Zinsmeister AR, Norton ID, Boardman LA, Devine RM. A comparison of endoscopic ultrasound, magnetic resonance imaging, and exam under anesthesia for evaluation of Crohn's perianal fistulas. Gastroenterology. 2001;121:1064-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 340] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 6. | van Bodegraven AA, Sloots CE, Felt-Bersma RJ, Meuwissen SG. Endosonographic evidence of persistence of Crohn's disease-associated fistulas after infliximab treatment, irrespective of clinical response. Dis Colon Rectum. 2002;45:39-45; discussion 45-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Bell SJ, Halligan S, Windsor AC, Williams AB, Wiesel P, Kamm MA. Response of fistulating Crohn's disease to infliximab treatment assessed by magnetic resonance imaging. Aliment Pharmacol Ther. 2003;17:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 129] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Van Assche G, Vanbeckevoort D, Bielen D, Coremans G, Aerden I, Noman M, D'Hoore A, Penninckx F, Marchal G, Cornillie F. Magnetic resonance imaging of the effects of infliximab on perianal fistulizing Crohn's disease. Am J Gastroenterol. 2003;98:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 251] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 9. | Roche B, Deléaval J, Fransioli A, Marti MC. Comparison of transanal and external perineal ultrasonography. Eur Radiol. 2001;11:1165-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Bernstein I, Juul N, Grønvall S, Bonde B, Klarskov P. Pelvic floor muscle thickness measured by perineal ultrasonography. Scand J Urol Nephrol Suppl. 1991;137:131-133. [PubMed] |
| 11. | Cornelia L, Stephan B, Michel B, Antoine W, Felix K. Trans-perineal versus endo-anal ultrasound in the detection of anal sphincter tears. Eur J Obstet Gynecol Reprod Biol. 2002;103:79-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Kiilholma PJ, Mäkinen JI, Pitkänen YA, Varpula MJ. Perineal ultrasound: an alternative for radiography for evaluating stress urinary incontinence in females. Ann Chir Gynaecol Suppl. 1994;208:43-45. [PubMed] |
| 13. | Piloni V. Dynamic imaging of pelvic floor with transperineal sonography. Tech Coloproctol. 2001;5:103-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Pregazzi R, Sartore A, Bortoli P, Grimaldi E, Troiano L, Guaschino S. Perineal ultrasound evaluation of urethral angle and bladder neck mobility in women with stress urinary incontinence. BJOG. 2002;109:821-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Schaer GN, Koechli OR, Schuessler B, Haller U. Perineal ultra-sound for evaluating the bladder neck in urinary stress incontinence. Obstet Gynecol. 1995;85:220-224. [DOI] [Full Text] |
| 16. | Rubens DJ, Strang JG, Bogineni-Misra S, Wexler IE. Transperineal sonography of the rectum: anatomy and pathology revealed by sonography compared with CT and MR imaging. AJR Am J Roentgenol. 1998;170:637-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Stewart LK, McGee J, Wilson SR. Transperineal and transvaginal sonography of perianal inflammatory disease. AJR Am J Roentgenol. 2001;177:627-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg. 1976;63:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1072] [Cited by in RCA: 919] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 19. | Mallouhi A, Bonatti H, Peer S, Lugger P, Conrad F, Bodner G. Detection and characterization of perianal inflammatory disease: accuracy of transperineal combined gray scale and color Doppler sonography. J Ultrasound Med. 2004;23:19-27. [PubMed] |
| 20. | Chew SS, Yang JL, Newstead GL, Douglas PR. Anal fistula: Levovist-enhanced endoanal ultrasound: a pilot study. Dis Colon Rectum. 2003;46:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Kruskal JB, Kane RA, Morrin MM. Peroxide-enhanced anal endosonography: technique, image interpretation, and clinical applications. Radiographics. 2001;21 Spec No:S173-S189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Poen AC, Felt-Bersma RJ, Eijsbouts QA, Cuesta MA, Meuwissen SG. Hydrogen peroxide-enhanced transanal ultrasound in the assessment of fistula-in-ano. Dis Colon Rectum. 1998;41:1147-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 89] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Sloots CE, Felt-Bersma RJ, Poen AC, Cuesta MA, Meuwissen SG. Assessment and classification of fistula-in-ano in patients with Crohn's disease by hydrogen peroxide enhanced transanal ultrasound. Int J Colorectal Dis. 2001;16:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |