Published online Oct 1, 2004. doi: 10.3748/wjg.v10.i19.2854
Revised: October 5, 2003
Accepted: October 12, 2003
Published online: October 1, 2004
AIM: Living related liver transplantation (LRLT) has been developed in response to the paediatric organ donor shortage. Though it has been succeeded in many centers worldwide, the safety of the donor is still a major concern, especially in donors with anatomy variation. We succeeded in performing the first two cases of living related liver transplantation with complicated anatomy of blood vessels as a way to overcome cadaveric organ shortage in Beijing.
METHODS: Two patients, with congenital liver fibrosis and congenital biliary atresia were performed with living donor liver transplantation in our hospital and then followed up from November 12 to December 13, 2001. The two living donors, mother and father, were healthy aged 34 and 35 years. One right lobe (segment V, VI, VII, VIII) and one left lateral lobe (segment II and III) were used. The grafts weighed 394 g and 300 g. The ratio of graft weight to the standard liver volume (SLV) of donors was 68% and 27%. The graft weight to recipient body weight ratio was 3.2% and 4.4%. The graft weight to recipient estimated standard liver mass (ESLM) ratio was 63% and 85%. The two donors had complicated blood vessel variation.
RESULTS: Two patients undergone living donor liver transplantation had good results. Abnormal liver function with high bilirubin level appeared in a few days after operation, but liver function returned to normal one month after operation with bilirubin level almost decreased to near normal. No bleeding, thrombosis, infection and bile leakage occurred. One had an acute rejection and recovered. The two donors recovered in two weeks. One had slight fever because of a little collection in abdomen and recovered after paracentesis and drainage.
CONCLUSION: Living donor liver transplantation has been proved to be a good way that offers a unique opportunity of getting a timely liver graft as a response to shortage of pediatric donors, though it could be a technically difficult operation if there is anatomical variation.
- Citation: Wu WH, Wan YL, Lee L, Yang YM, Huang YT, Chen CL, Fan ST. First two cases of living related liver transplantation with complicated anatomy of blood vessels in Beijing. World J Gastroenterol 2004; 10(19): 2854-2858
- URL: https://www.wjgnet.com/1007-9327/full/v10/i19/2854.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i19.2854
Orthotopic liver transplantation is an effective therapy for end-stage liver disease and has been proved to be a major advance in the treatment of children with liver disease. But the development of liver transplantation in infants and young children has been hampered by the shortage of donors, especially the scarcity of size-matched donors. Though reduced-size liver transplantation (RLT) was used in some centers, it does not expand the pool of potential donors and the mortality rate of recipients on the waiting list has remained high.
From November 12 to December 13 of 2001, the first two cases of living related liver transplantation in Beijing were successfully performed in our hospital. The donors were mother and father. The recipients were their 12-year-old and 31-month-old daughters. The donors and recipients all recovered about two months after operation. These two cases indicate the potential benefit of living related liver transplantation in Beijing.
Between November 12 and December 13 of 2001, living related liver transplantation was successfully performed on two cases in the First Hospital, Peking University. They were two girls. Underlying diseases were congenital liver fibrosis and congenital biliary atresia.
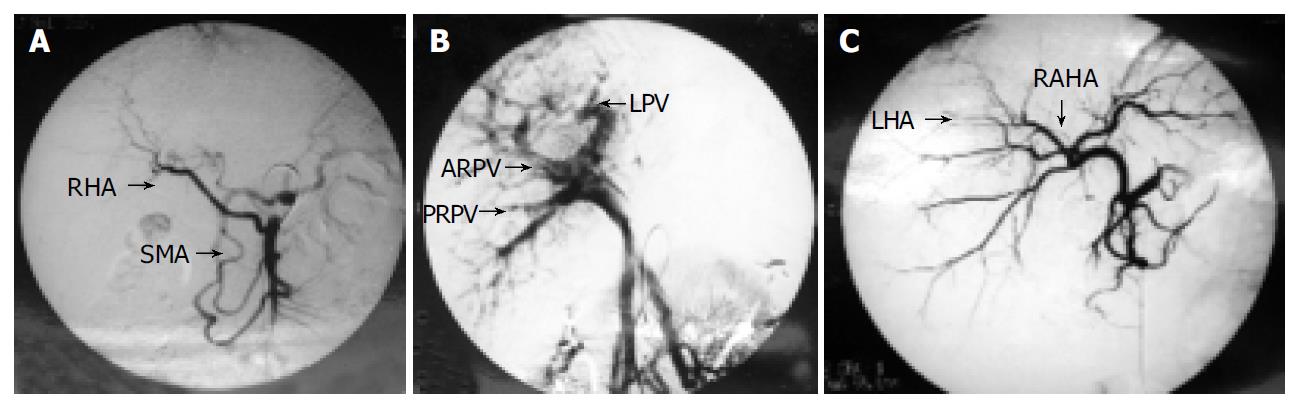
The living donors were mother and father with ABO identical blood group to the patients. There was no biochemical or serologic evidence of liver disease. Computerized tomography (CT) with volumetric measurement was performed to assess the volume of the right lobe and the left lateral lobe in order to determine the graft to be harvested. Angiographies to assess the vascular anatomy were performed in both donors. We found that both donors had blood vessel variations. In donor 1, the right hepatic artery (RHA) was from the superior mesenteric artery (SMA) (Figure 1A). The two branches of right portal veins (anterior and posterior right portal vein, ARPV and PRPV) of the mother were combined to the left portal veins (LPV) separately inside the liver parenchyma, no common trunk of right portal vein was found (Figure 1B). In donor 2, the left hepatic artery was derived from the right anterior hepatic artery, which was nearly inside the liver parenchyma (Figure 1C).
Laparotomy was performed via bilateral subcostal incision with an upward midline extension to the xiphoid process. Cholecystectomy was performed and the cystic duct was cannulated for cholangiography using undiluted radiographic contrast in order to delineate the ductal anatomy.
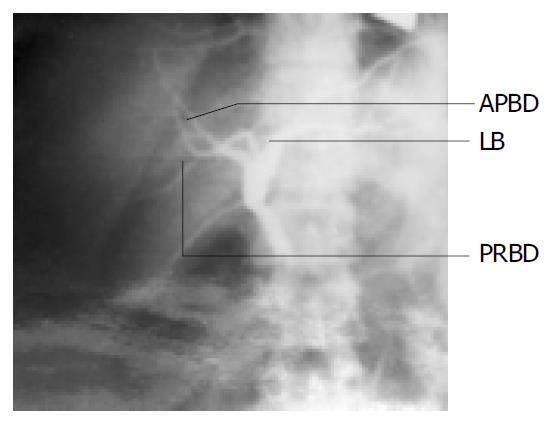
In donor 1, the cholangiography showed that it had the similar variation to the portal vein, the two branches of right bile ducts (anterior and posterior right bile ducts, ARBD and PRBD) of the mother were combined to the left bile duct separately which were inside the liver parenchyma (Figure 2).
Intraoperative ultrasonography was then performed to determine the configuration of the right hepatic vein, the junction of the middle hepatic vein with the left hepatic vein and to mark on the liver surface the position of the middle hepatic vein, which would be the liver transection plane. No right inferior hepatic veins were found in these two donors. The transection line was determined by the optimal graft volume and the anatomy of its drainage veins. The entire drainage area of the right and left hepatic veins were also identified by intra-operative ultrasonography. All of the right and left hepatic venous branches were traced to the periphery in the medial segment and marked with electrocautery on the liver surface. Hilar dissection was performed to free the right hepatic artery and portal vein. Branches of the right and left portal veins supplying the caudate process were divided and ligated.
In donor 1, dissection of the right portal veins (ARPV and PRPV) and the right hepatic ducts (ARBD and PRBD) was not made until the liver was transected, because they were inside the liver parenchyma due to anatomical variation. Parenchymal transection was performed by intraoperative ultrasonography with an ultrasonic dissector without vascular occlusion. The right hepatic ducts (the anterior and posterior ducts) were divided near the confluence of the bile ducts. The defect on the left hepatic duct was closed with 5-0 PDS monofilament absorbable sutures. After mobilization of the right liver, the right portal veins (the anterior and posterior veins), the right hepatic artery (from superior mesenteric artery) and vein were clamped at the junction with the main trunks and divided. At the back table, the graft was immersed in ice sludge and flushed with University of Wisconsin (UW) solution through the right portal venous cannula. The stumps of the right hepatic artery, the right hepatic vein and portal vein on the donor liver remnant were sutured.
In donor 2, The left hepatic artery, portal vein and hepatic vein were freed and hepatic parenchymal transection was accomplished using an ultrasonic dissector without vascular occlusion. Because the left hepatic artery was from the anterior right hepatic artery, the dissection of the left hepatic artery was partly inside the liver parenchyma when the liver transection was finished. The left duct was divided close to the cut surface of the liver, with an intact Glissonian sheath. The left hepatic duct was divided near the confluence of the bile ducts. The isolated graft was perfused in situ with the left portal vein clamped and cannulated by a catheter connected with cold lactated Ringer’s solution followed by a cold UW solution, when the left hepatic vein was opened.
The abdomen was entered through a bilateral subcostal incision with midline extension. Hilar dissection was performed to isolate and divide branches of the hepatic artery and portal vein. Then, total hepatectomy was performed with the inferior vena cava preserved.
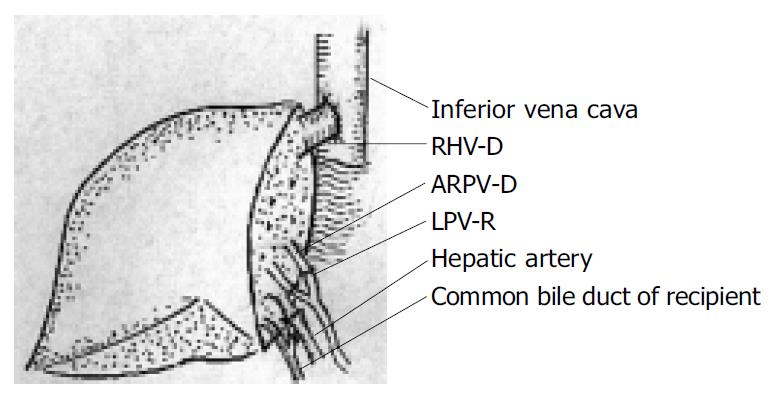
In recipient 1, the graft right hepatic vein (RHV-D) was anastomosed end-to-end to the recipient right hepatic vein (RHV-R). The graft anterior right portal vein (ARPV-D) was anastomosed end-to-end to the recipient left portal vein (LPV-R) and the graft posterior right portal vein (PRPV-D) was anastomosed end-to-end to the recipient right portal vein (RPV-R). The similar method was used to reconstruct the bile duct without stenting after the hepatic artery anastomosis (Figure 3).
In recipient 2, the graft left hepatic vein was anastomosed end-to-end to the confluence of the recipient left and middle hepatic veins. The graft left portal vein was anastomosed end-to-end to the truncated bifurcation of the recipient portal vein. The left hepatic duct was anastomosed to the Roux-en-Y limb of jejunum with an internal stent after the hepatic artery anastomosis.
The hepatic artery of the graft was anastomosed to the recipient hepatic artery using microvascular techniques.
Induction and maintenance immunosuppressive therapy consisted of a triple-drug regimen of steroid, cyclosporine A, and azathioprine. Acute rejection episode suspected on clinical and biochemical features and confirmed by percutaneous liver biopsy, was treated by intravenous bolus of methyl-predimetholone.
There was no operative mortality in the two living donors. One donor had postoperative complication of abdominal collection about two weeks after operation and recovered by percutaneous drainage about three weeks afteroperation. Blood losses during the donor operations were 150 mL and 70 mL respectively, and no blood transfusion was required in these two donor operations. Blood losses during the recipient operations were 50 mL and 100 mL respectively, and recipient 2 received 200 mL blood transfusion in the operation. The first warm ischaemia time of the grafts was 30 s and almost 0 s. The cold ischaemia time of the grafts was 120 min and 180 min. The second warm ischaemia time of the graft was 40 min and 50 min. The portal and arterial reperfusion interval time was 70 min and 90 min.
The weight of grafts was 394 g and 300 g, respectively. The ratio of graft weight to the standard liver volume (SLV) of donor was 68% and 27%, respectively. The graft weight to recipient body weight ratio was 3.2% and 4.4% respectively, and the graft weight to recipient estimated standard liver mass (ESLM) was 63% and 85%.
The duration of postoperative hospital stay of the donors was 7 d and 21 d. Most laboratory profiles returned to normal about two weeks after operation. The two donors had normal liver function and were able to return to their preoperative activities 3 mo after the operation.
Both patient and graft survived well 3 mo after operation. No postoperative complication occurred in recipient 1. Recipient 2 had an attack of acute rejection one week after the operation, and recovered after bolus treatment of methylprednisolone (1 g methylprednisolone per day for three days, iv).
Data on the postoperative serum total bilirubin (TBIL), direct bilirubin (DBIL), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and prothrombin activities (PTA) are shown in Table 1, Table 2, Table 3, Table 4.
| Content | Pre- | 1 d post- | 3 d post- | 7 d post- | 14 d post- |
| operation | operation | operation | operation | operation | |
| ALT (IU/L) | 12 | 372 | 110 | 48 | 45 |
| AST (IU/L) | 9 | 404 | 105 | 43 | 40 |
| TBIL (µg/L) | 10.5 | 80.2 | 32.5 | 7.4 | 8.2 |
| DBIL (µg/L) | 3.8 | 37.5 | 4 | 3.94 | 2.1 |
| PTA (%) | 78.0 | 32.0 | 29.0 | 56.0 | 72.0 |
| Content | Pre- | 1 d post- | 3 d post- | 7 d post- | 14 d post- |
| operation | operation | operation | operation | operation | |
| ALT (IU/L) | 37 | 816 | 305 | 42 | |
| AST (IU/L) | 30 | 817 | 58 | 37 | |
| TBIL (µg/L) | 7.7 | 21.8 | 9.7 | 5.0 | |
| DBIL (µg/L) | 3.5 | 3.24 | 2.3 | 0.78 | |
| PTA (%) | 82 | 127 | 151 | 127 |
| Content | Pre- | 1 d post- | 3 d post- | 7 d post- | 14 d post- | 21 d post- | 28 d post- |
| operation | operation | operation | operation | operation | operation | operation | |
| ALT (IU/L) | 40 | 372 | 189 | 103 | 80 | 24 | 28 |
| AST (IU/L) | 44 | 418 | 78 | 53 | 19 | 17 | 22 |
| TBIL (µg/L) | 17.4 | 73.4 | 63.3 | 80.6 | 58.6 | 53.6 | 23.8 |
| DBIL (µg/L) | 5.0 | 45.28 | 30.6 | 46.6 | 30.2 | 26.6 | 6.6 |
| PTA (%) | 89 | 18 | 31 | 43 | 41 | 73 | 81 |
| Content | Pre- | 1 d post- | 3 d post- | 7 d post- | 14 d post- | 21 d post- | 28 d post- |
| operation | operation | operation | operation | operation | operation | operation | |
| ALT (IU/L) | 105 | 1054 | 1 397 | 496 | 185 | 47 | 42 |
| AST (IU/L) | 250 | 3028 | 414 | 107 | 70 | 35 | 31 |
| TBIL (µg/L) | 480.4 | 87.7 | 209.3 | 216.2 | 205.4 | 96.7 | 44.2 |
| DBIL (µg/L) | 373.8 | 44.79 | 124.4 | 178.1 | 161.1 | 82.1 | 30.7 |
| PTA (%) | 92 | 7 | 16 | 58 | 72 | 76 | 78 |
The well-known inverse relationship between the availability of cadaveric pediatric organs and the epidemiology of pediatric liver disease has resulted in a substantial mortality rate among children on the waiting list[1,2]. Although RLT could alleviate the shortage of small donor organs, it did not expand the pool of potential donors, but induced a shift of grafts from a limited pool of adult cadaveric donors to pediatric recipients[3]. The use of liver donors has providesd a means for increasing the overall supply of livers[4,5].
Though liver consists of paired structures anatomically, safe separation of liver into its right and left halves requires considerable experience[6,7]. Fan et al[14] identified the reasons for unsatisfactory outcomes such as the presence of necrotic tissue on the liver transection surface, inadequate hepatic venous drainage, missed right posterior hepatic duct, and more than one right hepatic duct orifice on the graft. All these made its reconstruction difficult[8]. We followed the procedure “living donor liver transplantation using right lobe graft” by preferring the division of liver parenchyma along the Cantlie line[9]. To define the Cantlie line, we used the middle hepatic vein as a reference by using the intraoperative ultrasonogram[10,11]. To reduce the bleeding in the operation, we also decreased the central venous pressure to about 5 cm H2O[12].
The blood supply to the hepatic duct is dependent on a peribiliary plexus[13]. In the harvesting of the right or the left lobe graft, the blood supply is mainly from tiny arterial arcades from the right or the left portion of the caudate lobe[13]. So, the liver tissue cranial to the hepatic duct was cleared by ultrasonic dissector down to the caudate process and care was taken to avoid thinning the wall of the hepatic duct with ultrasonic dissector. We also noticed to maintain the blood pressure stable in the operation because hypotensive episodes would render the hepatic duct stump vulnerable to ischemia and necrosis[14].
In our group, the two donors had complicated anatomy variation. In donor 1, ARPV and PRPV were parallelly combined to the left portal vein, which were inside the liver parenchyma, no common trunk of right portal vein was found in extraparenchyma of the liver. The right hepatic duct had the similar anatomical variation with two parallel branches. This was rare in our past experiences and made the operation quite complicated. A series of 990-arterioportographic studies and 3000-endoscopic retrograde cholangio-pancreatic-graphic studies of the liver showed that the incidence of this kind of portal vein variation and hepatic bile duct variation was 0.15% and 5.08%, respectively[15-17]. When we tried to expose the ARPV and PRPV, we did not follow the main trunk of portal vein from the hilum, but dissected the hepatic parenchyma with intraoperative ultrasonography by an ultrasonic dissector without vascular occlusion in combination with hilar dissection to free the ARPV and PRPV[11]. Dissection of right hepatic ducts was not carried out until the liver was transected. Chen et al[4] reported that 83.33% intrahepatic ducts had the similar anatomy variation as the portal veins[18]. We paid more attention to intraoperative cholangiography and certified the similar anatomical variation in our group. We performed the same procedure to finish the dissection of anterior right hepatic duct and posterior right hepatic vein[19,20].
In donor 2, angiographic study showed that the left hepatic artery was from the anterior right hepatic artery. To avoid ischemia due to excessive hepatic artery dissection, which might cause biliary stricture or leakage[21], we dissected the left hepatic artery just on the left side of the common hepatic duct and carefully preserved the posterior right hepatic artery from the left side of the left hepatic artery. We also performed a little dissection in liver parenchyma with an ultrasonic dissector to get enough length of left hepatic artery for anastomosis[4]. It is important not to isolate the left hepatic artery too far into the left side of the liver parenchyma to avoid devascularization of the left hepatic duct[22].
Since these are the first two cases of living related liver transplantation in Beijing, ethical issues need to be considered when one contemplates liver transplantation from parent to child. These issues are similar to those associated with the transplantation of renal grafts from living related donors[23]. With the experience in liver resection, we conclude that the surgical risk is low enough to be acceptable for a parent or other close relatives if they volunteer to be an organ donor. Sure, it is an ethical dilemma of subjecting a healthy person to major hepatic resection to obtain a graft for a patient with end-stage liver disease[24-26]. We all agree with the congsensus that living-donor liver transplantation is not justified when there are sufficient cadaveric donors. However, the procedure could be justified for a patient with fulminant hepatic failure when no cadaver donor was available and when the recipient had a reasonable chance of a successful outcome[27].
The success of these first two cases has led to the acceptance of living related liver transplantation (LRLT) for clinical application in our center. With the excellent results in many centers and our present study[28,29], we consider that LRLT should be a good way for the care of patients with end-stage liver diseases or fulminant liver failure, particular in countries where cadaveric organ donation is limited or not available[30,31]. Though it might be very difficult technically, we should not give it up when we consider safety of the donor as our absolute priority first.
Edited by Xu JY and Wang XL Proofread by Xu FM
| 1. | Kelly D, Sibal A. Current status of liver transplantation. Indian J Pediatr. 2003;70:731-736. [PubMed] [DOI] [Full Text] |
| 2. | Ogura Y, Kaihara S, Haga H, Kozaki K, Ueda M, Oike F, Fujimoto Y, Ogawa K, Tanaka K. Outcomes for pediatric liver retransplantation from living donors. Transplantation. 2003;76:943-948. [PubMed] [DOI] [Full Text] |
| 3. | Abouljoud M, Yoshida A, Dagher F, Moonka D, Brown K. Living donor and split-liver transplantation: an overview. Transplant Proc. 2003;35:2772-2774. [PubMed] [DOI] [Full Text] |
| 4. | Chen CL, Chen YS, Liu PP, Chiang YC, Cheng YF, Huang TL, Eng HL. Living related donor liver transplantation. J Gastroenterol Hepatol. 1997;12:S342-S345. [PubMed] [DOI] [Full Text] |
| 5. | Malagó M, Testa G, Frilling A, Nadalin S, Valentin-Gamazo C, Paul A, Lang H, Treichel U, Cicinnati V, Gerken G. Right living donor liver transplantation: an option for adult patients: single institution experience with 74 patients. Ann Surg. 2003;238:853-62; discussion 862-3. [PubMed] [DOI] [Full Text] |
| 6. | Kamel IR, Lawler LP, Fishman EK. Variations in anatomy of the middle hepatic vein and their impact on formal right hepatectomy. Abdom Imaging. 2003;28:668-674. [PubMed] [DOI] [Full Text] |
| 7. | Yersiz H, Renz JF, Farmer DG, Hisatake GM, McDiarmid SV, Busuttil RW. One hundred in situ split-liver transplantations: a single-center experience. Ann Surg. 2003;238:496-505; discussion 506-7. [PubMed] [DOI] [Full Text] |
| 8. | Fan ST, Lo CM, Liu CL. Technical refinement in adult-to-adult living donor liver transplantation using right lobe graft. Ann Surg. 2000;231:126-131. [PubMed] [DOI] [Full Text] |
| 9. | Akamatsu N, Sugawara Y, Kaneko J, Sano K, Imamura H, Kokudo N, Makuuchi M. Effects of middle hepatic vein reconstruction on right liver graft regeneration. Transplantation. 2003;76:832-837. [PubMed] [DOI] [Full Text] |
| 10. | Cheng YF, Huang TL, Chen CL, Chen TY, Huang CC, Ko SF, Lee TY. Variations of the left and middle hepatic veins: application in living related hepatic transplantation. J Clin Ultrasound. 1996;24:11-16. [PubMed] [DOI] [Full Text] |
| 11. | Ko EY, Kim TK, Kim PN, Kim AY, Ha HK, Lee MG. Hepatic vein stenosis after living donor liver transplantation: evaluation with Doppler US. Radiology. 2003;229:806-810. [PubMed] [DOI] [Full Text] |
| 12. | Johnson M, Mannar R, Wu AV. Correlation between blood loss and inferior vena caval pressure during liver resection. Br J Surg. 1998;85:188-190. [PubMed] [DOI] [Full Text] |
| 13. | Stapleton GN, Hickman R, Terblanche J. Blood supply of the right and left hepatic ducts. Br J Surg. 1998;85:202-207. [PubMed] [DOI] [Full Text] |
| 14. | Fan ST, Lai EC, Lo CM, Chu KM, Liu CL, Wong J. Hepatectomy with an ultrasonic dissector for hepatocellular carcinoma. Br J Surg. 1996;83:117-120. [PubMed] [DOI] [Full Text] |
| 15. | Cheng YF, Huang TL, Lee TY, Chen TY, Chen CL. Variation of the intrahepatic portal vein; angiographic demonstration and application in living-related hepatic transplantation. Transplant Proc. 1996;28:1667-1668. [PubMed] |
| 16. | Huang TL, Cheng YF, Chen CL, Chen TY, Lee TY. Variants of the bile ducts: clinical application in the potential donor of living-related hepatic transplantation. Transplant Proc. 1996;28:1669-1670. [PubMed] |
| 17. | Kornasiewicz O, Krawczyk M, Paluszkiewicz R, Zieniewicz K, Hevelke P, Grzelak I, Pacho R, Rowiński O, Kaliciński P, Kaminski A. Anatomical alteration of the vascular tree observed during living related liver transplantation. Transplant Proc. 2003;35:2245-2247. [PubMed] [DOI] [Full Text] |
| 18. | Cheng YF, Huang TL, Chen CL, Sheen-Chen SM, Lui CC, Chen TY, Lee TY. Anatomic dissociation between the intrahepatic bile duct and portal vein: risk factors for left hepatectomy. World J Surg. 1997;21:297-300. [PubMed] [DOI] [Full Text] |
| 19. | Kornberg A, Heyne J, Schotte U, Hommann M, Scheele J. Hepatic venous outflow reconstruction in right lobe living-donor liver graft using recipient's superficial femoral vein. Am J Transplant. 2003;3:1444-1447. [PubMed] [DOI] [Full Text] |
| 20. | Hisatsune H, Yazumi S, Egawa H, Asada M, Hasegawa K, Kodama Y, Okazaki K, Itoh K, Takakuwa H, Tanaka K. Endoscopic management of biliary strictures after duct-to-duct biliary reconstruction in right-lobe living-donor liver transplantation. Transplantation. 2003;76:810-815. [PubMed] [DOI] [Full Text] |
| 21. | Sanchez-Urdazpal L, Gores GJ, Ward EM, Maus TP, Buckel EG, Steers JL, Wiesner RH, Krom RAF. Diagnostic features and clinical outcome of ischemic-type biliary complications after liver transplantation. Hepatology. 1993;17:605-609. [DOI] [Full Text] |
| 22. | Kanazawa A, Kubo S, Tanaka H, Takemura S, Yamazaki K, Hirohashi K, Shiomi S. Bile leakage after living donor liver transplantation demonstrated with hepatobiliary scan using 9mTc-PMT. Ann Nucl Med. 2003;17:507-509. [PubMed] [DOI] [Full Text] |
| 23. | D'Alessandro AM, Sollinger HW, Knechtle SJ, Kalayoglu M, Kisken WA, Uehling DT, Moon TD, Messing EM, Bruskewitz RC, Pirsch JD. Living related and unrelated donors for kidney transplantation. A 28-year experience. Ann Surg. 1995;222:353-362; discussion 362-364. [PubMed] [DOI] [Full Text] |
| 24. | Broelsch CE, Whitington PF, Emond JC, Heffron TG, Thistlethwaite JR, Stevens L, Piper J, Whitington SH, Lichtor JL. Liver transplantation in children from living related donors. Surgical techniques and results. Ann Surg. 1991;214:428-437; discussion 437-439. [PubMed] [DOI] [Full Text] |
| 25. | Basaran O, Karakayali H, Emiroğlu R, Tezel E, Moray G, Haberal M. Donor safety and quality of life after left hepatic lobe donation in living-donor liver transplantation. Transplant Proc. 2003;35:2768-2769. [PubMed] [DOI] [Full Text] |
| 26. | Lan AK, Luk HN, Goto S, Chen SM, Eng HL, Chen YS, de Villa VH, Wang CC, Cheng YF, Chen CL. Stress response to hepatectomy in patients with a healthy or a diseased liver. World J Surg. 2003;27:761-764. [PubMed] [DOI] [Full Text] |
| 27. | Majno P, Mentha G, Berney T, Bühler LH, Giostra E, Gelez P, Morard I, Bednarkiewicz M, Huber O, Morel P. Adult-to-adult living-donor liver transplantation. A summary of current status and an outline of the program in Geneva. Swiss Surg. 2003;9:227-236. [PubMed] [DOI] [Full Text] |
| 28. | Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, Takayama T, Makuuchi M. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198-1206; discussion 1206. [PubMed] [DOI] [Full Text] |
| 29. | Ghobrial RM, Busuttil RW. Future of adult living donor liver transplantation. Liver Transpl. 2003;9:S73-S79. [PubMed] [DOI] [Full Text] |
| 30. | Zimmerman MA, Trotter JF. Living donor liver transplantation in patients with hepatitis C. Liver Transpl. 2003;9:S52-S57. [PubMed] [DOI] [Full Text] |
| 31. | Sugawara Y, Makuuchi M, Kaneko J, Akamatsu N, Imamura H, Kokudo N. Living donor liver transplantation for hepatitis B cirrhosis. Liver Transpl. 2003;9:1181-1184. [PubMed] [DOI] [Full Text] |