INTRODUCTION
Many natural drugs for liver diseases are currently used in popular medicine. For example, quaternary protoberberine alkaloids from Flissitigma and Goniothalamus have been used in popular medicine for hepatomegaly and hepatosple-nomegaly[1,2]. The use of alkaloids from Berberis aristata for liver injury induced by chemical carcinogenesis and alkaloids from Enantica for disorders of bilirubin has also been reported[3,4].
Berberine is an isoquinoline alkaloid with a long history in both Ayurvedic and Chinese medicine. It exists in Hydrastis canadensis (golden seal), Coptis chinensis (Coptis or golden thread), Berberis aquifolium (Oregon grape), Berberis vulgaris (barberry), and Berberis aristata (tree turmeric). Berberine alkaloid can be found in roots, rhizomes, and stem bark of plants[5]. Berberine has been extensively studied and is known to exhibit multiple pharmacological activities such as antiprotozoal, antihypertensive[6], antibacterial[7], anti-inflammatory, anticholinergic[8] and antiarrhythmic activities[9]. Hwang et al[10] reported that berberine inhibited hepatotoxicity induced by tert-butyl hydroperoxide (t-BHP) via its antioxidant potential and could function as a chemopreventive agent in living systems. Previous studies have shown that berberine could block delayed rectifier potassium currents, inward rectifier potassium currents (IK1) and L-type calcium currents (ICa,L) in guinea pig ventricular myocytes[11,12]. So far, however, its hepatoprotective mechanism still remains unknown. No data are available on the relationship between ion currents in hepatocytes and the hepatoprotective effect of berberine.
Therefore, this study used patch-clamp techniques to record the whole-cell currents in isolated rat hepatocytes in order to investigate the hepatoprotective mechanism of berberine.
MATERIALS AND METHODS
Cell preparation
Rat hepatocytes were enzymatically isolated from Sprague-Dawley (SD) rats of either sex (150-200 g) by using slightly modified procedures described previously[13]. Briefly, adult animals were anesthetized with an intraperitoneal injection of pentobarbital sodium (30 mg/kg) in strict accordance to the guidelines established by the Institutional Animal Care and Use Committee, which follow all applicable state and federal laws. Portal vein and inferior vena cava were cannulated. The liver was initially perfused at a flow rate of 25 mL/min via a constant-flow system with modified oxygenated Ca2+, Mg2+-free Hanks’ solution for several minutes, followed by perfusion with a Ca2+, Mg2+-free Hanks’ solution containing collagenase (0.3 g/L, type I) for 10 min. The solutions were gassed with 950 mL/L O2 + 50 mL/L CO2 and heated to 37 °C. After these perfusions, the liver was excised and then minced in Ca2+, Mg2+-free Hanks’ solution at 0 °C. The cells were filtered through a 200 μm nylon mesh, and washed 3 times by centrifugation at 50 g for 2 min. The cell pellets were resuspended in KB medium that yielded approximately 85% to 95% viable hepatocytes. A small aliquot of the medium containing single cells was transferred into a 1-mL chamber mounted on the stage of an inverted microscope (IX-70, Olympus, Japan). Spherical and smooth cells were used for the whole-cell patch-clamp studies. All experiments were performed at room temperature (20 °C to 22 °C).
Voltage-clamp recording
A programmable vertical puller (pp-83, Narishige, Japan) was used to pull the electrodes. The resistance of capillary glass electrodes (GC150TF-10, Clark Electromedical Instruments, UK) used was 2 to 4 MΩ when filled with internal solution. A patch-clamp amplifier (EPC-9, Germany) was used to record the whole-cell currents with four-pole Bessel filter set at 1 kHz, digitized at 5 kHz. The protocols for voltage-clamp and data analysis were established with routines using pClamp 6.0 software (Axon Instrument, USA), and data were stored in computer for subsequent analysis. Drug actions were measured only after steady-state conditions reached, which were judged by the amplitudes and time courses of currents remaining constant with further perfusion of drugs.
Drugs and solutions
Berberine hydrochloride was obtained from Yichang Pharmaceutical Company of China as base powders, dissolved in distilled water and made into a stock solution at 0.1 mol/L. Berberine was added to bath solutions for extracellular application. All drugs were purchased from Sigma (USA) unless otherwise indicated.
Ca2+, Mg2+-free Hanks’ solution for cell-isolation (mmol/L) contained NaCl 137, KCl 5.4, NaH2PO4 0.5, Na2HPO4 0.58, NaHCO3 4.16 and glucose 5.5 (pH7.3). Kraft-bruhe (KB) medium for cell-preservation (mmol/L) contained L-glutamic acid 70, KCl 130, taurine 15, KH2PO4 10, MgCl2 0.5, glucose 11, N-(hydroxyethyl) piperazine-N’-2-ethanesulphonic acid (HEPES) 10 and ethylene glycol-O-O’-bis (2-aminoethyl) -N,N,N’,N’-tetraacetic acid (EGTA) 0.5 (pH7.4).
In studies of IK, the bath solution was a modified Tyrode’s solution (mmol/L) containing NaCl 144, KCl 4.0, CaCl2 1.8, MgCl2 0.53, Na2HPO4 0.33, HEPES 5 and glucose 5.5 (pH7.3). The patch pipette solution contained (mmol/L) KCl 130, K2ATP 5.0, creatine phosphate 5.0 and HEPES 5.0 (pH7.4).
For experiments on IK1, both the bath solution and the pipette solution contained (mmol/L) KCl 7, MgCl2 2, EGTA 1, K-glutamate 130 and HEPES 10 (pH7.4).
For ICRAC recording, the bath solution (mmol/L) contained NaCl 140, KCl 2.8 CaCl2 10, MgCl2 0.5, glucose 11 and HEPES 10 (pH7.4). The pipette solution used (mmol/L) contained K-glutamate 145, NaCl 8, MgCl2 1, MgATP 0.5, EGTA 10 and HEPES 10 (pH7.2).
Data analysis
All the data were expressed as mean ± SD and error bars were plotted as SD. Statistical significance was evaluated by a t test. Statistical differences were considered to be significant when P value was less than 0.05.
RESULTS
Effects of berberine on IK
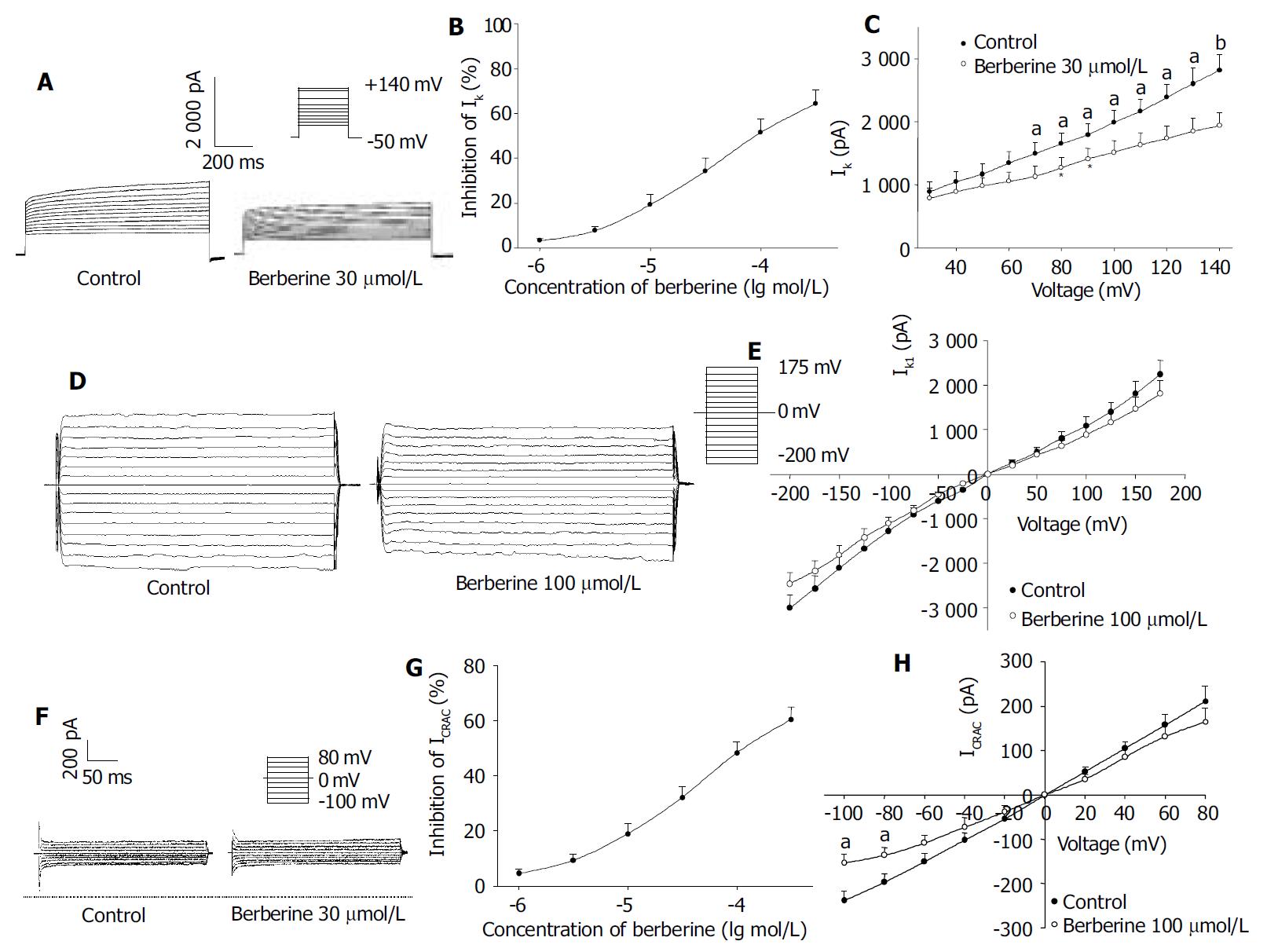
Under conventional whole-cell patch-clamp mode, the membrane potential was clamped at -50 mV, and IK was elicited in isolated rat hepatocytes by depolarizing pulse to +140 mV for 900 ms. The current at the end point of the test pulse was measured as the amplitude of IK.
The percentage block of IK was defined as (IControl-Iberberine)/ IControl and plotted as a function of logarithm [berberine] in Figure 1 B. At +140 mV, berberine 1-300 μmol/L decreased the IK amplitude concentration-dependently in all cells tested, which was poorly reversible after washout. The data points were fitted according to the Hill equation: Inhibition of current (%) = 100 / [1 + (EC50/C) nH], and an EC50 value of 38.86 ± 5.37 μmol/L and nH of 0.82 ± 0.05 were obtained ( n = 8). When the bath solution was changed to tetraethylammonium (TEA) 8 mmol/L, IK was inhibited.
Figure 1 Effects of berberine on IK.
A: Families of IK recorded with changes in the absence or presence of berberine 30 μmol/L and 100 μmol/L. Dotted line indicates zero current level; B: Dose-response curve for effects of berberine on IK; C: I-V relationship of IK under control (●) and berberine 30 μmol/L (○). D: Families of IK1 recorded with changes in the absence or presence of berberine 100 μmol/L. Dotted line indicates zero current level; E: I-V relationship of IK1 under control (●) and berberine 100 μmol/L (○). F: Families of ICRAC recorded with changes in the absence or presence of berberine 30 μmol/L. Dotted line indicates zero current level; G: Dose-response curve for effects of berberine on ICRAC; H: I-V relationship of ICRAC under control (●) and berberine 30 μmol/L (○). The voltage steps used to elicit IK are shown in the inset. n = 8, mean ± SD, aP < 0.05, bP < 0.01 vs control.
Figure 1 A shows the effects of berberine 30 μmol/L on the steady-state I-V relationship for IK generated by applying 12 steps voltage command pulse from +30 mV to +140 mV for 900 ms with a 10 mV increment from a holding potential of -50 mV. By comparing these two I-V curves shown in Figure 1 C, IK was reduced by berberine 30 μmol/L at all membrane potentials examined, especially at potentials positive to +60 mV (n = 8, P < 0.05 or P < 0.01 vs control). The results clearly indicated that berberine had a depressant action on IK in rat hepatocytes.
Effects of berberine on IK1
Hyperpolarizing and depolarizing potentials over a range from -200 mV to +175 mV with a 25 mV increment for 40 ms were applied from a holding level of 0 mV. The absolute value at the end of test pulse was measured as the amplitude of IK1. After administration of berberine 100, 300 μmol/L, the IK1 amplitude at -200 mV was decreased from -3044.75 ± 262.06 pA to -2451.33 ± 226.45 pA and -2310.66 ± 182.65 pA, respectively. The inhibition rates were 17.49% ± 2.51% and 24.11% ± 3.60%, respectively. Figure 1D and 1E shows the I-V relations of berberine 100 μmol/L on IK1. As shown in Figure 1D and 1E, berberine only had mild inhibitory effects on IK1 in isolated rat hepatocytes.
Effects of berberine on ICRAC
When the holding potential was 0 mV, and the cells were depolarized to -100 mV for 200 ms at a frequency of 0.2 Hz, ICRAC was evoked. As shown in Figure 1 G, ICRAC also was blocked by berberine in a concentration-dependent fashion, with an EC50 value of 47.20 ± 10.86 μmol/L and nH of 0.71 ± 0.09 ( n = 8). Figure 1 H shows the effect of berberine 30 μmol/L on the steady-stated I-V relationships generated by applying a series depolarizing pulses from a holding potential of 0 mV to different membrane potentials (-100 mV to +80 mV) with a 20 mV increment. The peak value of ICRAC was reduced on either inward or outward components, especially from -100 mV through -80 mV (n = 8, P < 0.05 vs control). But the reverse potential of ICRAC occurred at voltage 0 mV in all cells.
DISCUSSION
In this study, for the first time, we characterized the effects of berberine on IK, IK1 and ICRAC by patch-clamp techniques and demonstrated that berberine mainly inhibited IK, ICRAC and IK1 in isolated rat hepatocytes.
Potassium channels are ubiquitous in eukaryotic cells and could play roles in resting membrane potential, frequency of action potential, membrane potential repolarization rates and cell functions. It has been found that membrane potential is important in regulating metabolic processes in the liver, including gluconeogenesis, amino acid transport, and the rate of uptake of bile salts[14,15]. Changes in K+ permeability could affect the transmembrane potential. Bile formation was involved in anion accumulation within the apical lumen of hepatocytes. Potassium flux through hepatocellular basolateral membrane channels might provide the counterion for apical anion efflux[16]. Transcellular bile acid transport could be integrated in the regulation of intracellular pH, K+ homeostasis and membrane potential. Hepatocellular K+-depletion could result in the inhibition of bile acid secretion despite increasing the intracellular concentration[17-19].
During ischemia and hypoxia, hepatocellular volume and K+ conductance were increased and would lead to cell death[20,21]. Nietsch et al[22] demonstrated TNF (25 ng/mL) elicited a 2- and 5-fold increase in K+ current in hepatoma tissue culture cells. K+ channel activation might participate in pathways that leading to TNF-mediated cell death, thus representing potential therapeutic targets to attenuate liver injury from TNF. The inhibition of K+ channels could delay hepatocyte apoptosis and death. Churchill et al[23] reported that potassium channel antagonists had protective effects on rat liver via regulating the energy metabolism.
Previous studies showed that berberine (0.5, 5 mg/kg, i.p.) protected rat liver from hepatotoxicity induced by t-BHP[10]. As shown previously, t-BHP could shrink hepatocytes by release of cellular K+. Single-channel patch-clamp studies demonstrated t-BHP activated a 35-pS K+ channel and contributed to the K+ release of hepatocytes following exposure to t-BHP[24]. In this study, we observed that the concentration of berberine on IK was consistent with the dose reported by Hwang[10]. The hepatoprotective effect of berberine on t-BHP may have some relations with the inhibitory effect on potassium channel.
However, as we reported previously, AP-Q had a protective effect on CCl4-induced liver injury, probably by selectively increasing IK. In this experiment, we observed that berberine had a protective effect on t-BHP induced liver injury by decreasing IK. The contradiction may be due to the different mechanism of CCl4-induced and t-BHP induced liver injury. CCl4-induced hepatocyte injury paralleled with membrane depolarization by blocking the potassium channel in damaged hepatocytes[25-27]. However, t-BHP-induced liver injury is concerned with the release of cellular K+ by activating potassium channel. A previous study showed that berberine had not any protective effects on CCl4-induced liver injury[28].
Calcium has been demonstrated to play an important role in hepatocyte damage. An increase in calcium ion concentration in cytoplasm due to the influence of various toxic agents caused disturbances in the structure and function of hepatocytes, leading to their damage and even death[29]. Previous studies showed that hepatocellular Ca2+ overload and impaired Ca2+ signaling were related to hemorrhage/resuscitation liver injury. Increased Ca2+ uptake could result from a receptor-gated Ca2+ influx and/or oxygen-free radical induced membrane Ca2+ leaks. A protective effect of calcium channel blockers on hepatotoxins has been reported[30].
Calcium ions could enter the cells mostly through calcium channels. However, hepatocytes as the nonexcitable cells were short of the voltage-dependent Ca2+ channels but possessed plasma membrane Ca2+ channels that had a high selectivity for Ca2+, and were activated by a decrease in the concentration of Ca2+ in intracellular stores, which was named ICRAC[31,32]. Berberine inhibited ICRAC with EC50 of 47.20 μmol/L, which was different from the EC50 of ICa,L in cardiac myocytes[33]. The differential drug sensitivity of the two currents also provided further support for the idea that ICRAC is different from voltage-gated Ca2+ channel.
Berberine inhibited IK, IK1 and ICRAC concentration-dependently in isolated rat hepatocytes and the inhibitory extent was IK > ICRAC > IK1. Therefore, berberine could block K+ channel and decrease the extracellular K+ to regulate the metabolic processes in the liver. Berberine could also inhibit ICRAC effectively and protect hepatocytes from calcium overload via the inhibition of ICRAC. The inhibitory effects on potassium and calcium current may partly contribute to the hepatoprotective action of berberine. As berberine has already been used for liver damage in human beings[34], it may be a good candidate for further study and used in treatment of liver damage.