Published online Oct 1, 2004. doi: 10.3748/wjg.v10.i19.2836
Revised: April 10, 2004
Accepted: April 17, 2004
Published online: October 1, 2004
AIM: To examine the effects of tegaserod, a serotonin (5-HT) 4 receptor partial agonist, on abdominal withdrawal reflex (AWR) to rectal distention (RD) and c-Fos expression in limbic system.
METHODS: Neonatal Sprague-Dawley rats randomly received colonic irritation by acetic acid from postnatal day 8 to day 21 as a visceral hypersensitive model (group H) or by intrarectal saline as a control group (group C). When they became adults, rectal distention (RD) was performed by a balloon (6F; Fogarty arterial embolectomy catheter; length, 20 mm; diameter, 2 mm) which was rapidly inflated with increasing volumes of saline (0.4, 0.8 and 1.2 mL) for 20 s at five-minute intervals. Five subgroups of group H (H-saline, H-vehicle, H-Teg0.1, H-Teg0.3 and H-Teg1.0) were injected randomly with saline, vehicle (1-methyl-2-thpyrrolidone) or tegaserod at doses of 0.1, 0.3 and 1.0 mg/kg ip, respectively. Two subgroups of group C (C-Saline and C-Teg1.0) were injected with saline or tegaserod (1.0 mg/kg) ip. RD was performed 10 min after injection, AWR was recorded and c-Fos expression in limbic system was analyzed quantitatively by immunohistochemistry.
RESULTS: Compared to saline, tegaserod significantly inhibited AWR in group H (0.4 mL: from 2.0 to 0.5; 0.8 mL: from 3.5 to 1.5; 1.2 mL: from 4.0 to 3.0, P < 0.01), but had no significant effect on group C. Tegaserod dose-dependently attenuated the number of c-Fos positive neurons in limbic structures, anterior cingulate cortex (ACC) showed the greatest attenuation. In group H, tegaserod (1.0 mg/kg) resulted in a significant overall decrease to 57% of H-saline (283 ± 41 vs 162 ± 16, P < 0.01), in ACC to 42% of H-saline (72 ± 10 vs 31 ± 8, P < 0.01). In group C, tegaserod (1.0 mg/kg) resulted in an overall decrease to 77% of C-saline (214 ± 13 vs 164 ± 22, P < 0.01), in ACC to 65% of C-saline (48 ± 8 vs 31 ± 7, P < 0.01).
CONCLUSION: Tegaserod inhibits the response to rectal distention in rats with visceral hypersensitivity and dose-dependently attenuates c-Fos expression in limbic system, especially in anterior cingulate cortex.
- Citation: Jiao HM, Xie PY. Tegaserod inhibits noxious rectal distention induced responses and limbic system c-Fos expression in rats with visceral hypersensitivity. World J Gastroenterol 2004; 10(19): 2836-2841
- URL: https://www.wjgnet.com/1007-9327/full/v10/i19/2836.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i19.2836
Irritable bowel syndrome (IBS) is a common disorder characterized by abdominal pain and altered bowel habits, consisting of constipation, diarrhea, or both. Several pathophysiological mechanisms have been suggested to play a role in the genesis of symptoms in patients with IBS, among others visceral hypersensitivity, autonomic nervous system dysregulation[1], alterations of gastrointestinal (GI) motility[2], and abnormalities in neurotransmitter systems[3]. It has been shown that at least a subgroup of IBS patients shows a hyperalgesic response to visceral stimuli, and discomfort in response to colorectal balloon distension under experimental conditions[3,4]. Abnormalities which upregulate afferent (sensory) signal intensity anywhere in the “brain-gut axis” could induce visceral hypersensitivity[5].
It has been shown in experimental rats that rectal distention is a non-invasive, reproducible visceral stimulus, which can induce a range of pseudoaffective responses, including vasomotor, visceromotor, and respiratory responses[3]. Abdominal withdrawal reflex (AWR) is an involuntary motor reflex similar to the visceromotor reflex[6]. Intestinal distention can be considered to as an appropriate stimulus for studies of visceral nociception[7].
In previous studies, it has been shown that noxious distension of hollow viscera induces a specific pattern of c-Fos expression in rat limbic brain structures[3,8], which involved in higher cognitive functions (i.e. emotion, memory, motivation) and led to the perception of visceral pain[9]. Induction of c-Fos expression is a well established marker of neuronal activation, and immunohistological detection of c-Fos-like immunoreactivity allows a mapping of activated brain nuclei on a single cell level[3].
Serotonin (5-HT) is thought to play a role in visceral nociceptive mechanisms. There is considerable evidence that serotonin is involved in the regulation of motility and sensation in the gut[3]. In animal studies, tegaserod was reported to inhibit abdominal contraction response to noxious intestinal distention[10]. Tegaserod, a 5-HT4 receptor partial agonist, could relieve symptoms in irritable bowel syndrome patients with abdominal pain, bloating and constipation[11]. However, little is known about the effect of tegaserod on neuronal activity in limbic structures at noxious rectal distention. Therefore, in the present study, we established a rat model to investigate the role of 5-HT4 receptors in mediating activation of limbic structures at rectal distention, as assessed by c-Fos expression. We aimed to establish a mechanism of the action of 5-HT4 receptors specific to visceral nociceptive neurotransmission.
Experiments were performed using Sprague-Dawley rats obtained as preweanling neonates (younger than 8 d) from the Animal Center in the First Hospital of Peking University. Rats were housed in plastic cages containing corn chip bedding and maintained on a 12:12-h light-dark cycle (lights on at 7 AM) at 22 to 23 °C and in 60% - 65% humidity. The irritation procedure and the experimental testing were conducted during the light component of the cycle. The neonates were housed 12 in a cage with their mothers until they were 25 d old. Mothers had access to food and water ad libitum. After separation, the rats were housed 4 in a cage with access to food and water ad libitum. The animals were deprived of food but water 18 h before rectal distention (RD). Animal care and experimental procedures were followed institutional ethics guidelines and conformed to the requirements of the State Authority for Animal Research Conduct.
Each rat was weighed every 3 d from days 9 to 40.
Neonatal Sprague-Dawley rats (8 d old) were divided into 2 groups (group C: control and group H: hypersensitivity) undergoing different treatments. Forty-eight rats in group H received intracolonic injections of 5 mL/L acetic acid (0.5 mL) daily between the ages of 8 and 21 d. Acetic acid was injected into the colon via the PE90 tube inserted to 2 cm from the anus. Twenty-four rats in group C received intracolonic injections of 9 g/L saline (0.5 mL) daily between the ages of 8 and 21 d[12].
Because tegaserod (HTF 919; Novartis Pharma AG, Basel, Switzerland) is poorly soluble in water, the fractions were made up using 0.1 mL of 1-methyl-2-pyrrolidinone (vehicle)[10]. After dissolved in the vehicle, distilled water was added to make the solution up to 0.5 mL. According to the drugs injected intraperitoneally 10 min before RD, 48 rats in group H were divided into 6 subgroups (H0, H-saline, H-vehicle, H-Teg0.1, H-Teg0.3, and H-Teg1.0), and 24 rats in group C were divided into 3 subgroups (C0, C-saline, and C-Teg1.0), eight rats in each subgroup. Rats in group C-saline and H-saline were injected with saline (0.5 mL), in group C-Teg1.0 with tegaserod (1.0 mg/kg), in group H-vehicle with vehicle (0.1 mL and distilled water 0.4 mL) and in group H-Teg0.1, H-Teg0.3, and H-Teg1.0 with tegaserod at a dose of 0.1, 0.3 or 1.0 mg/kg, respectively. Groups H0 and C0 were not distended and only histological examination and myeloperoxidase (MPO) activity assay were done.
Three weeks after cessation of the irritation protocol, in groups H0 and C0, the distal 4-5 cm of the descending colon and rectum was removed and histological analysis and MPO activity assay were performed. MPO activity assay was performed as described previously[13,14]. MPO activity was expressed as U/g protein.
Behavioral responses to RD were assessed in all groups 3 wk after cessation of the irritation protocol by measuring the abdominal withdrawal reflex (AWR) using a semiquantitative score. AWR is an involuntary motor reflex similar to the visceromotor reflex. However, the advantage of AWR over the visceromotor reflex is that the latter requires additional surgery to implant recording electrodes and wires in the abdominal muscles, which may cause additional sensitization in an already sensitized system. Distention balloons (described below) were placed in the rectum of lightly sedated adult rats (ether) and secured by taping the attached tube to the rat’s tail. The rats were then housed in small Lucite cubicles (20 cm × 8 cm × 8 cm) on an elevated Plexiglas platform and allowed to wake up and adapt (20 min). Measurement of the AWR consisted of visual observation of animal response to graded RD (0.4, 0.8 and 1.2 mL) by blinded observers and assignment of AWR scores: 0, no behavioral response to RD; 1, brief head movement followed by immobility; 2, contraction of abdominal muscles; 3, lifting of abdomen; 4, body arching and lifting of pelvic structures. The rats were given RD for 20 s every 5 min. To achieve an accurate measure, distensions were repeated 5 times for each volume. The data for each animal were averaged for analysis. The results obtained were compared among groups. A change in the magnitude of an evoked response indicated a change in visceral pain processing.
Colon stimulation consisted of graded RD produced by inflating a balloon inside the rectum. The balloon, 2 cm in length and 2 mm in diameter (6F, Fogarty arterial embolectomy catheter, Baxter, USA), was carefully inserted intrarectally and fixed at a distance of 1 cm with an adhesive tape at the tail of the rat. Distension was produced by rapidly inflating the balloon to the desired volumes with saline (0.4, 0.8 or 1.2 mL) for 20 s at 5-min intervals. Before they were used, the balloons were blown up and left overnight so the latex stretched and the balloons became compliant. Tegaserod (0.1, 0.3 or 1.0 mg/kg) or saline or vehicle was administered 10 min prior to RD. Only a single dose was tested in each animal.
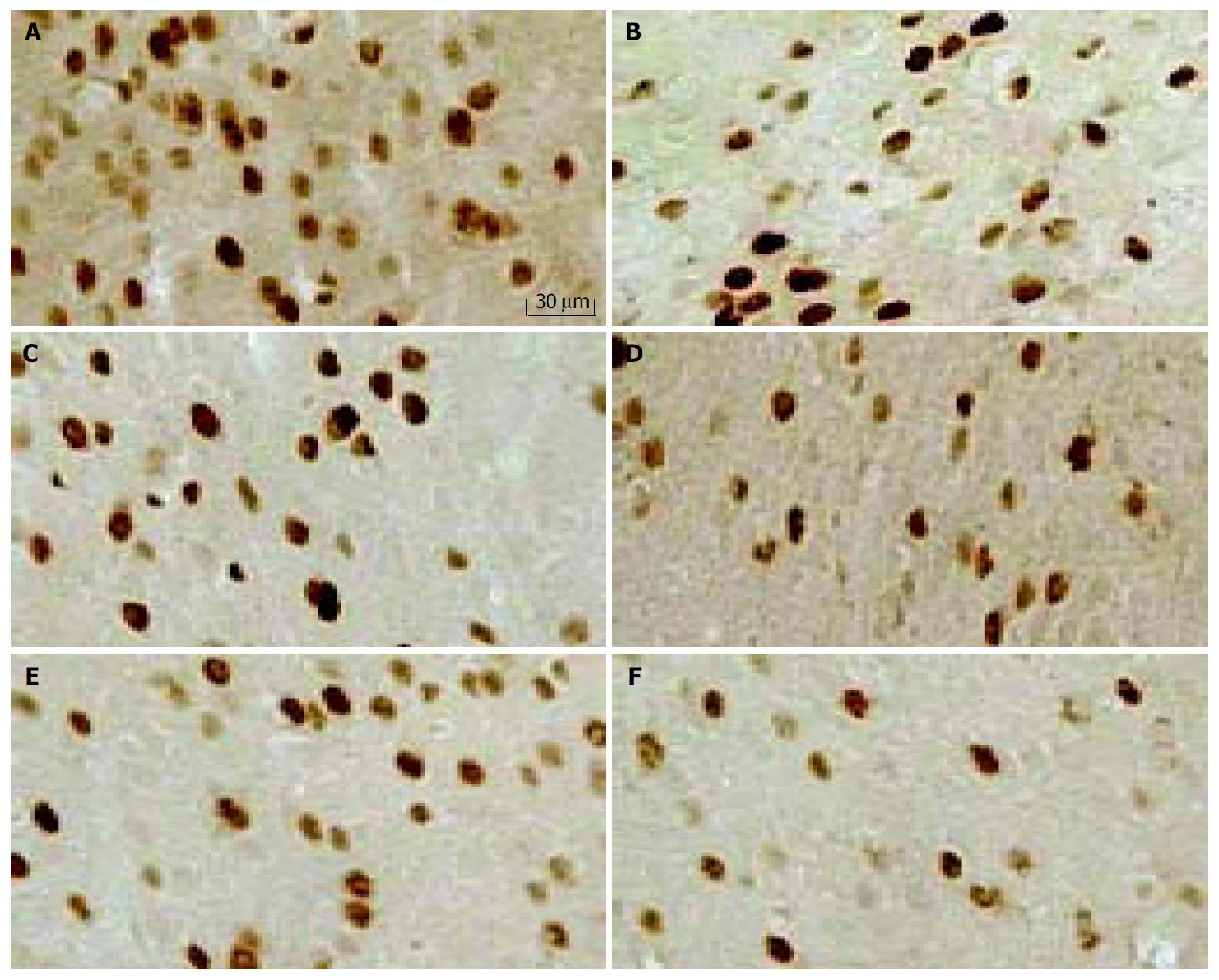
Within 30 min following the end of the distention procedure, the animals were deeply anesthetized with an overdose of sodium pentobarbitone (60 mg/kg intraperitoneally) and then perfused through the ascending aorta with saline (9 g/L), followed by 500 mL of cold 0.1 mol/L phosphate buffer (PB, 4 °C) containing 40 g/L paraformaldehyde (pH7.4). The brain was immediately removed and postfixed in the same fixative at 4 °C overnight, and then placed in 300 g/L sucrose with 0.1 mol/L PB for 72 h at 4 °C. Coronal sections (40 μm thick) were cut from frozen blocks at the levels of brain regions of interest (1.5 mm to 5 mm posterior to bregma, according to the atlas of Paxinos and Watson). Every fifth section was stained for c-Fos-like immunoreactivity (c-Fos-ir) using the method of free floating for immunohistochemistry. Briefly, sections were first washed 3 times in phosphate buffered saline plus 3 g/L Triton X-100 (PBS-T) (5 min each time) at room temperature, and incubated for 10 min with PBS-T containing 50 mL/L normal goat serum to block nonspecific binding sites and facilitate tissue penetration. Then sections were washed with PBS-T and incubated for 24 h at room temperature with PBS-T containing rabbit polyclonal anti-Fos protein antiserum (Zhongshan, China) (diluted 1:200). After washed with PBS-T, sections were incubated with biotinylated anti-rabbit IgG (1:300, Zhongshan, China) for 120 min. The sections were then incubated with strepta-vidin-peroxidase conjugate (1:300) for 120 min and subsequently visualized using diaminobenzidine (DAB) as chromogen. Sections were mounted on gelatin-coated glass slides, air dried, dehydrated in ethanol, and xylene, then coverslipped with DePeX. Brain sections were examined using bright-field microscopy. The same lot of antibody was used for each study outlined below. The primary c-Fos antibody was omitted in one well of each immunohistological reaction as a negative control. In each study, every staining process included free-floating sections of all groups using the same buffers and solutions.
The number of c-Fos-like immunoreactive (c-Fos-ir) nuclei was counted in 5 sections of each rat as identified by morphology using an image analysis package (Leica Q550CW running QWIN software; Leica UK Ltd, Milton Keynes, UK). In the anterior cingulate cortex (ACC), thalamus (TH), hippocampus (HP), hypothalamus (HypoTH) and amygdala (Amy), c-Fos-ir nuclei were counted individually and expressed as the number per 600 × 500 pixel. All brain regions were counted bilaterally in each section. The total number of c-Fos-ir nuclei in five sections was used for subsequent data analysis. The counts comprising all those nuclear immunoreactive signals could be clearly distinguished from the background.
Statistical analysis was done using SPSS for windows 11.0. The results of weight, MPO activity and the number of c-Fos-ir were expressed as mean ± SD, and statistical significances were determined using Student’s paired t test or one way analysis of variance (ANOVA), followed by Dunnett’s post hoc test. The median values of the AWR scores among all groups at each volume of RD were compared using the Mann-Whitney U-test. P < 0.05 was considered statistically significant.
Masses on d 9 and 40 were not statistically different between groups H and C (18.9 ± 3.2 g vs 19.6 ± 3.2 g, 154.4 ± 12.7 g vs 149.3 ± 16.5 g, respectively). The model did not alter the growth rate of the rats.
The identifiable histopathology was absent in the adult colons. The tissues showed no significant structural damage or loss of crypts. Mucin depletion or increase in intraepithelial lymphocytes was not seen in any of the tissues examined. MPO activity was not statistically different between groups H and C (20.49 ± 1.64 U/g protein and 17.49 ± 6.35 U/g protein, respectively).
| 0.4 mL | 0.8 mL | 1.2 mL | |
| C-saline | 1.0 (0-2) | 2.5 (2-3) | 3.0 (3-4) |
| C-Teg1.0 | 0.0 (0-2) | 2.0 (2-2) | 3.0 (3-4) |
| Z value | 1.465 | 2.236 | 0 |
| P value | 0.195 | 0.105 | 1.000 |
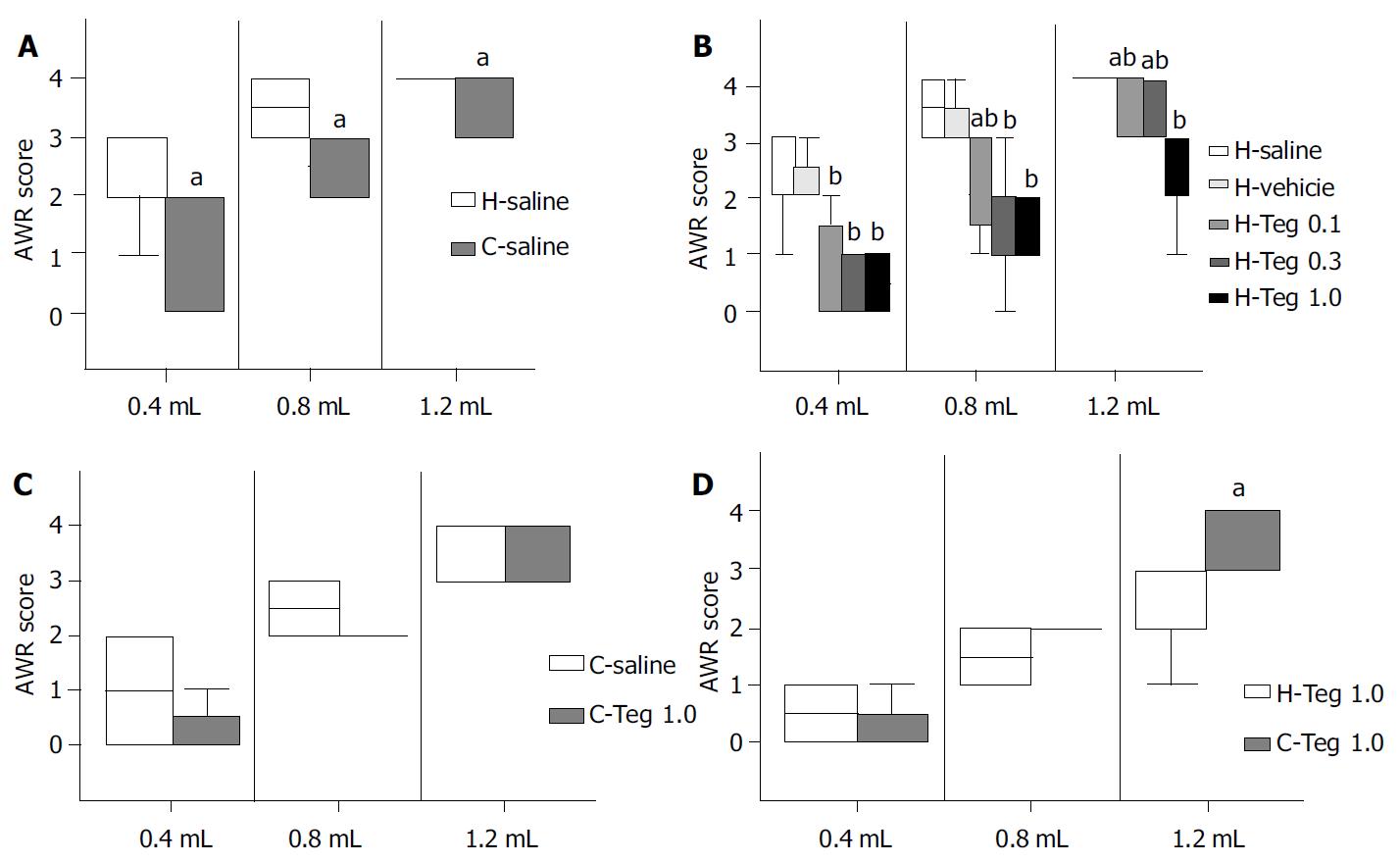
H-saline vs C-saline Median AWR scores at volumes of 0.4, 0.8, and 1.2 mL were significantly higher in H-saline than in C-saline (2.0, 3.5, and 4.0, vs 1.0, 2.5, and 4.0, respectively) (P < 0.05). These results suggested that the model of visceral hypersensitivity in this study was reliable (Figure 1 A).
AWR in subgroups of group H Median AWR scores were similar in H-saline and in H-vehicle, so the effect of vehicle could be negligible. AWR scores were significantly higher in H-saline than in H-Teg0.1, H-Teg0.3, and H-Teg1.0. The 5-HT4 receptor agonist tegaserod (0.1, 0.3, and 1.0 mg/kg) significantly inhibited the response to rectal distention in rats with hypersensitivity (P < 0.01). The difference between H-Teg0.1 and H-Teg0.3 was not significant at all volumes. The difference between H-Teg0.1 and H-Teg1.0 was significant at the volumes of 0.8 mL and 1.2 mL (P < 0.05). The difference between H-Teg0.3 and H-Teg1.0 was significant only at 1.2 mL (P < 0.05). At the volume of 0.4 mL, differences of AWR scores in H-Teg0.1, H-Teg0.3, and H-Teg1.0 were not significant (Figure 1 B, Table 1).
C-saline vs C-Teg1.0 Median AWR scores were similar in C-saline and in C-Teg1.0 at all volumes. These results suggested that tegaserod (1.0 mg/kg) had little effect on the AWR response to RD in control rats (Figure 1 C, Table 2).
C-Teg1.0 vs H-Teg1.0 At 1.2 mL, AWR scores were higher in C-Teg1.0 than in H -Teg1.0 (P < 0.05). While at 0.4 mL and 0.8 mL, the differences between H-Teg1.0 and C-Teg1.0 were not significant. It seemed that the inhibitory effect on AWR at largest volume (1.2 mL) was stronger in hypersensitive condition than in normal condition (Figure 1 D).
| ACC Hippocampus HypoTH Amygdala Thalamus Overall | ||||||
| H-saline | 72 ± 10 | 54 ± 13 | 51 ± 17 | 44 ± 16 | 62 ± 19 | 283 ± 41 |
| H-vehicle | 72 ± 10 | 54 ± 13 | 49 ± 15 | 43 ± 15 | 62 ± 19 | 281 ± 37 |
| H-Teg0.1 | 53 ± 14b | 48 ± 10 | 47 ± 13 | 42 ± 11 | 50 ± 13 | 239 ± 31a |
| H-Teg0.3 | 40 ± 11b | 45 ± 7 | 41 ± 11a | 37 ± 11 | 45 ± 11a | 208 ± 25b |
| H-Teg1.0 | 31 ± 8b | 36 ± 9b | 31 ± 10b | 30 ± 8b | 34 ± 10b | 162 ± 16b |
| F value | 60.583 | 11.605 | 8.199 | 5.362 | 14.574 | 61.989 |
| P value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
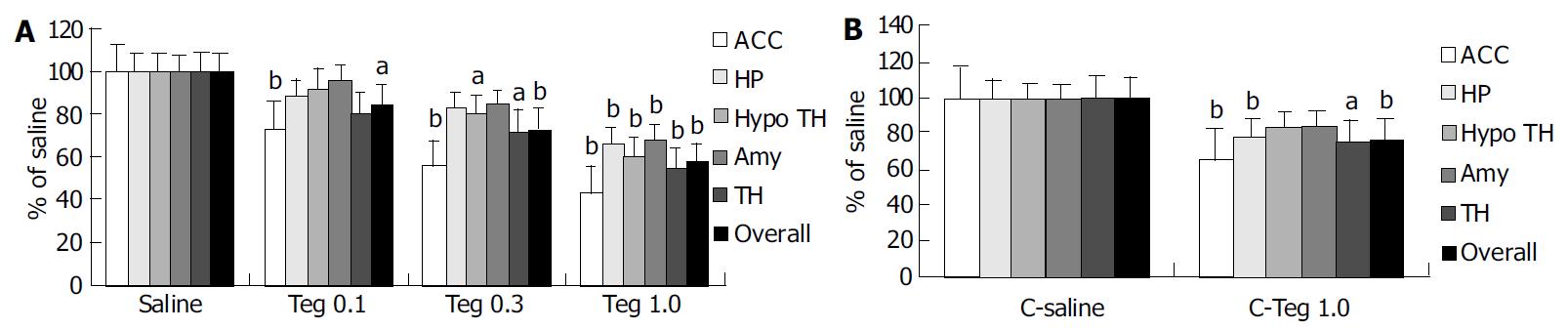
Repetitive RD significantly induced changes in neuronal activity in all animals, as determined by increased density of c-Fos-ir cells (Figure 2). c-Fos expression was located bilaterally in discrete areas of limbic structures. The overall number of c-Fos-ir cells in H-saline was significantly more than that in C-saline (283 ± 41 vs 214 ± 13, P < 0.001), but the difference was not significant in hippocampus (54 ± 13 vs 46 ± 7, P = 0.374). Tegaserod given ip 10 min prior to RD produced a significant, dose-dependent attenuation in the total number of c-Fos-ir nuclei in limbic brain compared with saline. These effects were especially clear in anterior cingulate cortex (ACC). In the hippocampus and amygdala, the effect of tegaserod was significant only at the high-dose (1.0 mg/kg). Tegaserod (0.1 mg/kg) decreased the overall c-Fos-ir to 85% of saline (P < 0.05). The greatest attenuation was in ACC (74% of H-saline), while amygdala was least affected (95% of H-saline). Tegaserod (0.3 mg/kg) significantly attenuated c-Fos expression in ACC, hypothalamus, and thalamus (to 56%, 80% and 73% of saline, respectively). A high dose of tegaserod (1.0 mg/kg) resulted in a significant (P < 0.01) overall decrease of c-Fos in five areas, the overall number was decreased to 57% of saline. Similar to the low dose, ACC showed the greatest attenuation (42% of H-saline), while other regions were decreased to approximately 60% of H-saline (Figure 3 A).
In group C, although the inhibitory effect of tegaserod on AWR was not significant, the attenuation effect on c-Fos expression was also observered. Tegaserod (1.0 mg/kg) resulted in a significant overall decrease in c-Fos to 77% of C-saline. Similarly, ACC also was the greatest attenuation (65% of C-saline). In addition, tegaserod could decrease c-Fos in thalamus and hippocampus (76% and 78% of C-saline, respectively) (Figure 3 B).
Experimental data suggested that patients with IBS had visceral sensory dysfunction so that physiological stimuli could induce their symptoms. Visceral afferent input is modulated by a variety of mechanisms operating between the gastrointestinal tract and the brain, and dysfunction of these regulatory mechanisms could distort gastrointestinal perception[16]. Intestinal discomfort reaches awareness via neural connections termed the “brain-gut axis”. Abnormalities which upregulate afferent (sensory) signal intensity anywhere in this system could induce hypersensitivity, pain, and discomfort. Several features of IBS suggest involvement of the brain’s emotional limbic system, such as higher prevalence of anxiety and psychosocial stressors, augmented intestinal and stress responses and response to centrally acting medication. Recent brain imaging data suggested that pathways involved in visceral pain perception overlapped with limbic pathway[17,18]. In the brain, increased thalamic activation has been seen in IBS, which could indicate increased afferent output from lower levels. Activation of the anterior cingulate cortex, the limbic center that encodes pain suffering, appeared to be enhanced in IBS, especially under the influence of anxiety[5].
Colonic irritation with acetic acid in neonatal rats could lead to a state of chronic visceral hypersensitivity in adults[19-21]. This model did not alter the growth rate of the rats. This hypersensitivity occurs in the absence of identifiable histopathology in the adult colon and does not change MPO activity of colonic tissue. Myeloperoxidase (MPO), a hydrogen peroxide (H2O2) oxidoreductase, is specifically found in mammalian granulocytic leukocytes, including polymorphonuclear leukocytes (PMNs), monocytes, basophils and eosinophils. MPO activity has been widely accepted as an enzyme marker to measure and quantitate the PMN content in a variety of tissues. It has been suggested that measurement of MPO provides a simple and specific method to quantitate PMN accumulation or infiltration in a variety of pathological processes accompanied with inflammation[22].
5-HT4 receptors have been found to be involved in regulating the sensitivity of rectal mechanoreceptive afferents[23]. Tegaserod is a 5-HT4 receptor partial agonist with a relatively long half-life (approximate 11 h in dogs and humans)[24]. It could dose-dependently inhibit the abdominal contraction response to noxious intestinal distention not linked to alterations in compliance[10]. In our study, in rats with hypersensitivity but not in control rats, tegaserod potently inhibited AWR at all volumes of distention. It seemed that the inhibitory effect on AWR at the highest volume (1.2 mL) was more powerful in hypersensitive condition than in normal condition. The results are inconsistent with the study of Coelho et al[10]. Two reasons may have been responsible for these phenomena. One reason might be that the patterns of intestinal distention were different. In the study of Coelho et al, the balloon was an inflated 5 min step of 15 mmHg, from 0 to 60 mmHg. The other reason might be that 5-HT4 receptors played a relatively modest role in modulating visceral sensation under basal physiological condition[25]. Thus, we tentatively suggest that the number of 5-HT4 receptors may be upregulated and/or the effect followed by the activity of 5-HT4 receptor may be increased under hypersensitive status. The exact mechanisms of its action need to be further researched.
c-fos, an immediate-early gene, could be expressed within the neurons following voltage-gated calcium entry into the cells[26]. c-fos was induced by noxious stimulus, generally as the result of an injury[27]. c-Fos protein encoded by c-fos, has been regarded as a third messenger molecule which couples the short term extracellular signals with the long term alteration in cell function when neurons are excited[28]. c-Fos could be interpreted as an increase in activity of those neurons expressing the protein[27], so it is usually used as a marker to indicate the activation of neurons[29]. Following injury there was a correlation between the expression of c-Fos and magnitude of hyperalgesia, and c-Fos expression following noxious intensities of intestinal distention could reflect the intensity of stimuli and the degree of discomfort[27]. Previous study has demonstrated that c-Fos is expressed in limbic brain structures in response to noxious rectal distention[9]. Limbic structures play an important role in visceral pain processing. The present report provided quantitative data on expression of c-Fos protein induced by rectal distention and the effect of tegaserod at different doses on c-Fos expression in brain nuclei, such as hypothalamus, thalamus, amygdala, hippocampus, and anterior cingulate cortex following noxious rectal distension. Functional brain imaging researches have demonstrated that colorectal distention could cause abnormal activation in ACC[17] and thalamus in IBS patients compared with control[17,18]. From our results, we conclude that tegaserod dose-dependently attenuates c-Fos expression in limbic structures. Especially in ACC and thalamus, the effect of tegaserod is more evident. This may do good for IBS patients.
5-HT4 receptors are widely distributed in peripheral and central sites. In gut, 5-HT4 receptors are located primarily on the enterochromaffin cells and less on smooth muscle cells, enterocytes, and neurons[24]. 5-HT4 receptors are highly expressed in several brain regions, such as limbic areas, periaqueductal grey matter and sensory terminals[30] and in spinal cord and dorsal root ganglion neurons[25]. The site of action of tegaserod was not established in the present study, but could be at the level of enteric or primary sensory neurons, or via spinal or supraspinal neuronal circuits concerned with the modulation of nociceptive transmission. A study by Schikowski et al[23] demonstrated that tegaserod might have a direct effect on the mechanoreceptive afferents. We supposed that 5-HT4 receptors might directly decrease the signals ascending to the central nervous system (CNS) or might decrease the activity of CNS (as demonstrated by c-Fos-ir nuclei), which would benefit for IBS patients. Little is known about the effect of tegaserod on response following intracerebroventricular injection. Further studies are needed to determine the central action of 5-HT4 receptors.
In conclusion, tegaserod inhibits response to noxious distention, and the effect is more evident in hypersensitive condition than in control. Tegaserod dose-dependently attenuates c-Fos expression in limbic structures, especially in anterior cingulate cortex. Therefore, tegaserod decreases central sensitization. Tegaserod may be of potential use in the treatment of visceral pains.
The authors are grateful to Professor Xin-Guang Liu and Hua-Hong Wang for advice, and to Novartis Pharmaceuticals Corp for providing the drugs for this study.
Edited by Kumar M and Wang XL Proofread by Xu FM
| 1. | Tougas G. Irritable bowel syndrome: new approaches to its pharmacological management. Can J Gastroenterol. 2001;15 Suppl B:12B-13B. [PubMed] |
| 2. | Mönnikes H, Tebbe JJ, Hildebrandt M, Arck P, Osmanoglou E, Rose M, Klapp B, Wiedenmann B, Heymann-Mönnikes I. Role of stress in functional gastrointestinal disorders. Evidence for stress-induced alterations in gastrointestinal motility and sensitivity. Dig Dis. 2001;19:201-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 189] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 3. | Mönnikes H, Rüter J, König M, Grote C, Kobelt P, Klapp BF, Arnold R, Wiedenmann B, Tebbe JJ. Differential induction of c-fos expression in brain nuclei by noxious and non-noxious colonic distension: role of afferent C-fibers and 5-HT3 receptors. Brain Res. 2003;966:253-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Miura M, Lawson DC, Clary EM, Mangel AW, Pappas TN. Central modulation of rectal distension-induced blood pressure changes by alosetron, a 5-HT3 receptor antagonist. Dig Dis Sci. 1999;44:20-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Mertz H. Role of the brain and sensory pathways in gastrointestinal sensory disorders in humans. Gut. 2002;51 Suppl 1:i29-i33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 554] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 7. | Ness TJ. Intravenous lidocaine inhibits visceral nociceptive reflexes and spinal neurons in the rat. Anesthesiology. 2000;92:1685-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Monnikes H, Lauer G, Arnold R. Peripheral administration of cholecystokinin activates c-fos expression in the locus coer-uleus/subcoeruleus nucleus, dorsal vagal complex and paraventricular nucleus via capsaicin-sensitive vagal afferents and CCK-A receptors in the rat. Brain Res. 1997;770:277-288. [RCA] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 143] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Traub RJ, Silva E, Gebhart GF, Solodkin A. Noxious colorectal distention induced-c-Fos protein in limbic brain structures in the rat. Neurosci Lett. 1996;215:165-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Coelho AM, Rovira P, Fioramonti J, Bueno L. Antinociceptive properties of HTF 919 (Tegaserod), a 5-HT4 receptor partial agonist, on colorectal distension in rats. Gastroenterology. 2000;118:A835. [DOI] [Full Text] |
| 11. | Müller-Lissner SA, Fumagalli I, Bardhan KD, Pace F, Pecher E, Nault B, Rüegg P. Tegaserod, a 5-HT(4) receptor partial agonist, relieves symptoms in irritable bowel syndrome patients with abdominal pain, bloating and constipation. Aliment Pharmacol Ther. 2001;15:1655-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 336] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 12. | Liu YB, Yuan YZ, Tao RJ, Zhai ZK, Chen HZ. Establishment of a rat model of gut hypersensitivity and for evaluation of vis-ceral sensitivity. Chin J Dig. 2003;23:34-37. |
| 13. | Al-Awadi FM, Khan I. Studies on purine enzymes in experimental colitis. Mol Cell Biochem. 1999;194:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189576] [Cited by in RCA: 157132] [Article Influence: 3206.8] [Reference Citation Analysis (0)] |
| 15. | Chowdhury GM, Fujioka T, Nakamura S. Induction and adaptation of Fos expression in the rat brain by two types of acute restraint stress. Brain Res Bull. 2000;52:171-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Azpiroz F. Hypersensitivity in functional gastrointestinal disorders. Gut. 2002;51 Suppl 1:i25-i28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Mertz H, Morgan V, Tanner G, Pickens D, Price R, Shyr Y, Kessler R. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology. 2000;118:842-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 400] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 18. | Yuan YZ, Tao RJ, Xu B, Sun J, Chen KM, Miao F, Zhang ZW, Xu JY. Functional brain imaging in irritable bowel syndrome with rectal balloon-distention by using fMRI. World J Gastroenterol. 2003;9:1356-1360. [PubMed] |
| 19. | Lin C, Al-Chaer ED. Long-term sensitization of primary afferents in adult rats exposed to neonatal colon pain. Brain Res. 2003;971:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Lidow MS, Song ZM, Ren K. Long-term effects of short-lasting early local inflammatory insult. Neuroreport. 2001;12:399-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Ruda MA, Ling QD, Hohmann AG, Peng YB, Tachibana T. Altered nociceptive neuronal circuits after neonatal peripheral inflammation. Science. 2000;289:628-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 286] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 22. | Xia Y, Zweier JL. Measurement of myeloperoxidase in leukocyte-containing tissues. Anal Biochem. 1997;245:93-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 122] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Schikowski A, Thewissen M, Mathis C, Ross HG, Enck P. Serotonin type-4 receptors modulate the sensitivity of intramural mechanoreceptive afferents of the cat rectum. Neurogastroenterol Motil. 2002;14:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Lacy BE, Yu S. Tegaserod: a new 5-HT4 agonist. J Clin Gastroenterol. 2002;34:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Bharucha AE, Camilleri M, Haydock S, Ferber I, Burton D, Cooper S, Tompson D, Fitzpatrick K, Higgins R, Zinsmeister AR. Effects of a serotonin 5-HT(4) receptor antagonist SB-207266 on gastrointestinal motor and sensory function in humans. Gut. 2000;47:667-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Premkumar DR, Adhikary G, Overholt JL, Simonson MS, Cherniack NS, Prabhakar NR. Intracellular pathways linking hypoxia to activation of c-fos and AP-1. Adv Exp Med Biol. 2000;475:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Traub RJ, Stitt S, Gebhart GF. Attenuation of c-Fos expression in the rat lumbosacral spinal cord by morphine or tramadol following noxious colorectal distention. Brain Res. 1995;701:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Saria A, Fischer HS, Humpel C, Pfattner A, Schatz DS, Schuligoi R. Margatoxin and iberiotoxin, two selective potassium channel inhibitors, induce c-fos like protein and mRNA in rat organotypic dorsal striatal slices. Amino Acids. 2000;19:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Harris JA. Using c-fos as a neural marker of pain. Brain Res Bull. 1998;45:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 390] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 30. | Espejo EF, Gil E. Antagonism of peripheral 5-HT4 receptors reduces visceral and cutaneous pain in mice, and induces visceral analgesia after simultaneous inactivation of 5-HT3 receptors. Brain Res. 1998;788:20-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |