Published online Sep 15, 2004. doi: 10.3748/wjg.v10.i18.2675
Revised: January 5, 2004
Accepted: January 12, 2004
Published online: September 15, 2004
AIM: To construct ltB-ureB fusion gene and its prokaryotic expression system and identify immunity and adjuvanticity of the expressed recombinant protein.
METHODS: The ureB gene from a clinical Helicobacter pylori (H pylori) strain Y06 and the ltB gene from Escherichia coli (E. coli) strain 44851 were linked into ltB-ureB fusion gene by PCR. The fusion gene sequence was analyzed after T-A cloning. A prokaryotic recombinant expression vector pET32a inserted with ltB-ureB fusion gene (pET32a-ltB-ureB) was constructed. Expression of the recombinant LTB-UreB protein (rLTB-UreB) in E. coli BL21DE3 induced by isopropylthio-β-D-galactoside (IPTG) at different concentrations was detected by SDS-PAGE. Western blot assays were used to examine the immunoreaction of rLTB-UreB by a commercial antibody against whole cell of H pylori and a self-prepared rabbit anti-rUreB serum, respectively, and determine the antigenicity of the recombinant protein on inducing specific antibody in rabbits. GM1-ELISA was used to demonstrate the adjuvanticity of rLTB-UreB. Immunoreaction of rLTB-UreB to the UreB antibody positive sera from 125 gastric patients was determined by using ELISA.
RESULTS: In comparison with the corresponding sequences of original genes, the nucleotide sequence homologies of the cloned ltB-ureB fusion gene were 100%. IPTG with different dosages of 0.1-1.0 mmol/L could efficiently induce pET32a-ltB-ureB-E. coli BL21DE3 to express the rLTB-UreB. The output of the target recombinant protein expressed by pET32a-ureB-E. coli BL21DE3 was approximately 35% of the total bacterial proteins. rLTB-UreB mainly presented in the form of inclusion body. Western blotting results demonstrated that rLTB-UreB could combine with the commercial antibody against whole cell of H pylori and anti-rUreB serum as well as induce rabbit to produce specific antibody. The strong ability of rLTB-UreB binding bovine GM1 indicated the existence of adjuvanticity of the recombinant protein. All the UreB antibody positive sera from the patients (125/125) were positive for rLTB-UreB.
CONCLUSION: A recombinant prokaryotic expression system with high expression efficiency of the target fusion gene ltB-ureB was successfully established. The expressed rLTB-UreB showed qualified immunogenicity, antigenicity and adjuvanticity. All the results mentioned above laid a firm foundation for further development of H pylori genetically engineered vaccine.
-
Citation: Yan J, Wang Y, Shao SH, Mao YF, Li HW, Luo YH. Construction of prokaryotic expression system of
ltB-ureB fusion gene and identification of the recombinant protein immunity and adjuvanticity. World J Gastroenterol 2004; 10(18): 2675-2679 - URL: https://www.wjgnet.com/1007-9327/full/v10/i18/2675.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i18.2675
In China, gastritis and peptic ulcer are the most prevalent gastric diseases and gastric cancer is one of the malignant tumors with high morbidities[1]. Helicobacter pylori (H pylori), a microaerophilic, spiral and Gram-negative bacterium, is recognized as a human-specific gastric pathogen that colonizes the stomachs of at least half of the world’s populations[2]. Most infected individuals are asymptomatic. However, in some subjects, H pylori infection causes acute, chronic gastritis or peptic ulceration. Furthermore, the infection is also a high risk factor for the development of peptic ulcer and gastric adenocarcinoma, mucosa-associated lymphoid tissue (MALT) lymphoma and primary gastric non-Hodgkin’s lymphoma[3-8]. Recently, direct evidence of carcinogenesis of the microbe in an animal model has been presented[9-11]. Immunization against the bacterium represents a cost-effective strategy to prevent H pylori-associated common peptic ulcer diseases and to reduce the incidence of global gastric cancers[12]. However, no vaccines preventing H pylori infection have been commercially available so far.
Previous studies revealed many protective protein antigens of the microbe such as UreB, HpaA, FlaA, CagA, VacA etc[13-18]. Among these protein antigens, UreB, one of the four subunits of an urease produced by almost all the isolated strains of H pylori, has been demonstrated to have the strongest antigenicity and protection in all known proteins of H pylori[13,19,20]. ureB gene, responsible for encoding UreB with 569 amino acid residues, is a highly conserved nucleotide sequence with a similarity of approximately 95% in different H pylori isolates[21-23]. These data strongly indicate that UreB can be used as an excellent antigen candidate for H pylori vaccine.
Since a genetically engineered vaccine composed of a single protein antigen usually showed a low immunization effect, it is necessary to increase immunogenicity of the antigen by co-administration with an appropriate adjuvant. Escherichia coli (E. coli) heat-labile toxin B subunit (LTB) and cholera toxin B subunit (CTB) were well-confirmed mucosal adjuvants[24-28]. However, some of the previous studies demonstrated that the mucosal adjuvanticity of LTB was stronger than that of CTB[26,29]. Furthermore, CTB activates Th2 pathway, and induces IL-4, a cytokine closely related to IgE-mediated allergic reaction, but LTB mainly stimulates Th1 pathway[26,30].
In order to simplify the procedure steps and further reduce cost in H pylori vaccine production, we constructed ltB-ureB fusion gene and its recombinant prokaryotic expression system. The immunogenicity, antigenicity and adjuvanticity of the expressed target recombinant protein (rLTB-UreB) were examined. The results of this study would benefit the mass production of H pylori UreB-associated genetically engineered vaccine at a lower cost.
Both the ureB gene from a clinical H pylori strain Y06 and the ltB gene from E. coli strain 44851 (offered by National Institute for the Control of Pharmaceuticals and Biological Products of China) was cloned by our laboratory[31]. A plasmid pET32a (Novagen, Madison, USA) and E. coli BL21DE3 (Novagen, Madison, USA) were used as the expression vector and host cell, respectively. Primers for PCR amplification were synthesized by BioAsia (Shanghai, China). Taq-plus high fidelity PCR kit and restriction endonucleases used were purchased from TaKaRa (Dalian, China). The T-A Cloning Kit, DNA Agarose Gel Purification Kit and sequencing service were provided by BBST (Shanghai, China). DAKO (Glostrup, Denmark) and Jackson ImmunoResearch (West Grove, USA) supplied rabbit antiserum against whole cell of H pylori, HRP-labeling sheep anti-rabbit IgG and anti-human IgG antibodies, respectively. The UreB antibody positive serum samples from 125 H pylori infected patients with gastritis or ulcer were stored at -70 °C in our laboratory[31].
Extraction of DNA templatesE. coli DH5α strains respectively containing plasmid pUCm-T-ureB, pUCm-T-ltB were cultured in LB medium. The two plasmids were extracted by alkaline-denature method and then purified by DNase-free RNase treatment and routine phenol-chloroform method described by Sambrook et al[32]. The obtained DNA extracts were dissolved in TE buffer and their concentrations as well as purity were measured by ultraviolet spectrophotometry[32]. The pUCm-T-ureB DNA was further digested with restriction endo-nucleases EcoR V and Xho I at 37 °C for 3 h. The target fragment of ureB gene was separated by agarose gel electrophoresis and then recovered by DNA Agarose Gel Purification Kit.
Amplification of ureB and ltB gene The sequence of ltB sense primer was: 5’-CCGGATATCATGAATAAAGTAAAATGTTA-3’(EcoR V). The sequence of antisense primer linking the 3’-end of ltB gene and the 5’-end of ureB gene was: 5’-AGAAACATATTCTTTTCTGCTAATGTTTTCCATACTGATTGCCGC-3’. Total volume per PCR was 100 µL containing 2.5 mol/L each dNTP, 250 nmol/L each of the two primers, 15 mol/L MgCl2, 2.5 U Taq-plus polymerase, 100 ng pUCm-T-ltB DNA template and 1 × PCR buffer (pH8.3). Parameters for PCR of ltB gene were: at 94 °C for 5 min, × 1; at 94 °C for 30 s, at 48 °C for 30 s, at 72 °C for 45 s, × 10; at 94 °C for 30 s, at 48 °C for 30 s, at 72 °C for 50 s (an addition of 5 s for each of the following cycles), × 20; finally at 72 °C for 7 min, × 1. The results of PCR were observed under UV light after electrophoresis in 15 g/L agarose pre-stained with ethidium bromide. The expected size of target amplification fragment from ltB gene was 375 bp. The target fragment in the gel was recovered by using DNA Agarose Gel Purification Kit.
Construction of ltB-ureB fusion gene by PCR Total volume per tube was 90 μL containing all the PCR reagents mentioned above but not the primers, 100 ng of the recovered ltB DNA fragment with a cohesive end and 400 ng of the recovered ureB DNA fragment were added. Parameters for the following PCR were: at 94 °C for 5 min, × 1; at 94 °C for 30 s, at 45 °C for 30 s, at 72 °C for 150 s, × 10; at 72 °C for 10 min, × 1. After this PCR, the two fragments of ureB and ltB produced a complex fragment of ureB-ltB dependent on the cohesive end in the ltB fragment, which would be used as a template for the next PCR. The sense primer for ureB-ltB amplification was as previously mentioned. The sequence of antisense primer was: 5’-CGACTCGAGGAAAATGCTAAAGAGTTGTGC-3’ (Xho I). The 250 nmol/L each of the two primers was added into each of the tubes. Parameters for ltB-ureB amplification were: at 94 °C for 3 min, × 1; at 94 °C for 30 s, at 50 °C for 30 s, at 72 °C for 180 s, × 10; at 94 °C for 30 s, at 50 °C for 30 s, at 72 °C for 195 s (an addition of 15 s for each of the following cycles), × 15; at 72 °C for 12 min, × 1. Examination of the results of this PCR and recovery of the target fragment were the same as described above. The expected size of target amplification fragment from ureB-ltB fusion gene was 2070 bp.
T-A cloning, sequencing and subcloning of ureB-ltB fusion gene The ltB-ureB amplification DNA fragment was cloned into plasmid vector pUCm-T (pUCm-T-ltB-ureB) by using T-A Cloning Kit according to the manufacturer’s instruction. The recombinant plasmid was amplified in E. coli DH5α and then extracted by Sambrook’s method[32]. A professional company (BBST) was responsible for nucleotide sequence analysis of the inserted fragment. Two plasmids pUCm-T-ltB-ureB and pET32a in two different strains of E. coli DH5α after amplified in LB medium were extracted and then digested with EcoR V and Xho I, respectively[32]. The fragment ltB-ureB and pET32a were recovered and then ligased. The recombinant expression vector pET32a-ltB-ureB was transformed into E. coli BL21DE3, and the expression system was named as pET32a-ltB-ureB-E. coli BL21DE3. The ltB-ureB fragment inserted in pET32a was sequenced again.
Expression of the target recombinant proteinpET32a-ltB-ureB-E. coli BL21DE3 was rotatively cultured in LB medium at 37 °C induced by isopropylthio-β-D-galactoside (IPTG) at different concentrations of 1.0, 0.5 and 0.1 mmol/L. The supernatant and precipitate were separated through centrifugation after the bacterial pellet was ultrasonically broken (300 V, 3 × 5 s). The molecular mass and output of the target recombinant protein (rLTB-UreB) were measured by SDS-PAGE.
Identification of immunoreactivity and antigenicity of rLTB-UreB The expressed rLTB-UreB was collected by Ni-NTA affinity chromatography. The commercial rabbit antiserum against whole cell of H pylori or rabbit anti-rUreB serum prepared in our previous study and HRP-labeling sheep anti-rabbit IgG were used as the first and second antibodies, respectively, to determine the immunoreactivity of rLTB-UreB by Western blot. Rabbits were immunized with rUreB to prepare the antiserum and Western blot was applied again to determine the antigenicity of rLTB-UreB.
GM1-ELISA GM1-ELISA was used to demonstrate the adjuvanticity of rLTB-UreB. Briefly, 40-well plates were coated by bovine GM1 (Sigma) and then added with rLTB-UreB. The rabbit anti-rLTB-UreB serum was used as the first antibody (1:100 dilution) and the commercial HRP-labeling sheep anti-human IgG (1:4000 dilution) was applied as the second antibody. Each of the first antibody dilutions contained four wells. Negative controls without addition of rLTB-UreB with four repeated wells were set up and their mean A490 value plus 3-fold SD values were used as the positive standard for each of the tested wells[33].
ELISA By using rLTB-UreB as coated antigen at the concentration of 20 μg/mL, each of the UreB antibody positive serum samples from the 125 patients (1:400 dilution) as the first antibody and HRP-labeling sheep anti-human IgG (1:4000 dilution) as the second antibody, the immunoreaction of rLTB-UreB to the specific antibody in the sera were detected by ELISA. In this assay, six UreB antibody negative serum samples were used as the control and the positive standard was similar to that in the GM1-ELISA.
The nucleotide sequence of the cloned ltB-ureB fusion gene was compared for homologies with the original sequences[31] by using a molecular biological analysis software.
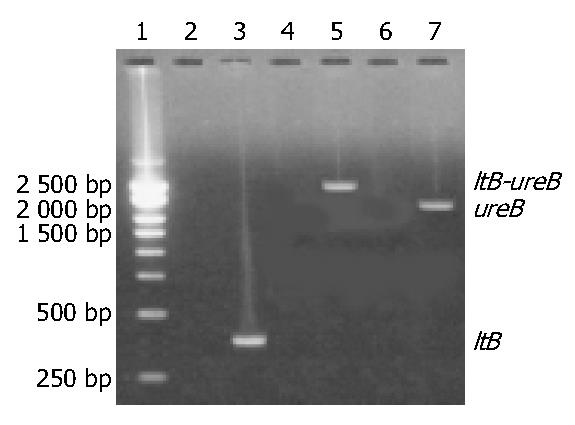
The target fragments of ureB, ltB and ltB-ureB genes with the expected sizes are shown in Figure 1.
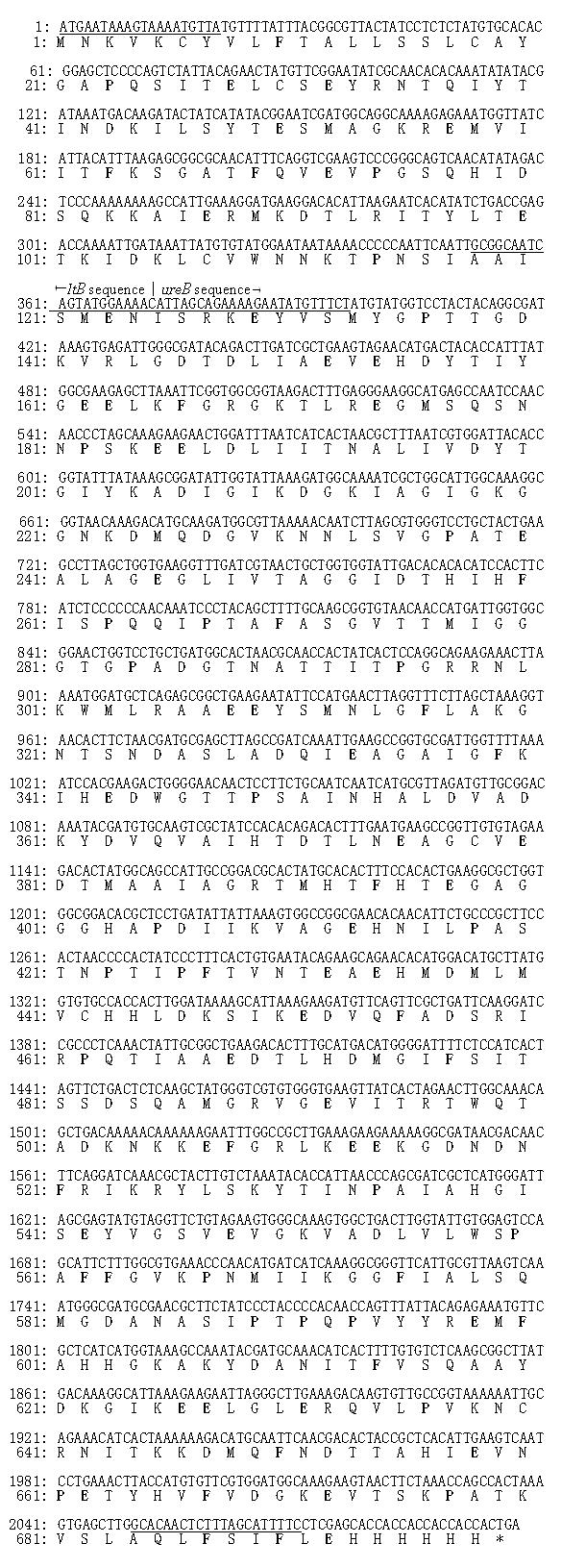
The homologies of the nucleotide sequences of the cloned ltB-ureB fusion gene compared with the original ltB and ureB gene sequences were 100%[31]. The nucleotide and putative amino acid sequences of the ltB-ureB fusion gene are shown in Figure 2.
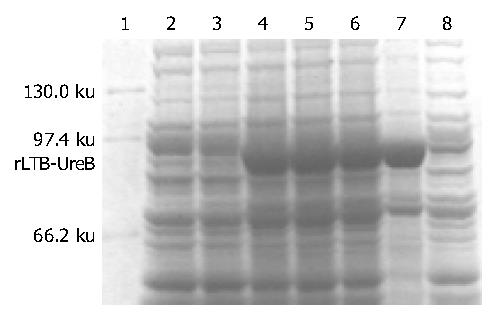
IPTG at concentrations of 1.0, 0.5 and 0.1 mmol/L could efficiently induce the expression of rLTB-UreB in pET32a-ltB-ureB-E. coli BL21DE3 system. The product of rLTB-UreB was mainly presented in the ultrasonic precipitate and the output was approximately 35% of the total bacterial proteins (Figure 3).
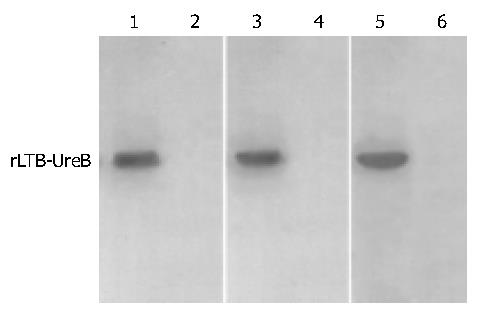
Commercial rabbit antibody against the whole cell of H pylori could combine with rLTB-UreB and induce rabbit to produce specific antibody as confirmed by Western blotting (Figure 4), respectively.
Since the mean ± SD of A490 of the negative control in the four repeated wells was 0.28 ± 0.09, the positive reference value was 0.55. The mean ± SD of A490 of the tested wells was 1.29 ± 0.10, indicating that rLTB-UreB had the ability of binding to bovine GM1.
Since the mean ± SD of A490 values of the six UreB antibody negative serum samples was 0.17 ± 0.03, the positive reference value for the specific antibody detection in patients’ sera was 0.26. According to the reference value, 100% (125/125) of the tested patients’ sera were positive for the antibodies against rLTB-UreB with an A490 value ranging from 0.37-1.98.
The selection of antigenic targets is critical in the design of H pylori vaccine. A large number of published data showed that UreB might be the most definitive antigen candidate for H pylori vaccine[13,19-23]. On the other hand, LTB is found to be the most efficient mucosal adjuvant with few possibility of inducing allergic reaction[24-30]. So UreB and LTB should be the optimal antigen and adjuvant for developing orally taken H pylori vaccine, respectively.
In the present study, ltB-ureB fusion gene was obtained by using three PCRs and the nucleotide sequence of the gene showed absolutely the same as the corresponding ones. This data indicated that the method used for constructing fusion gene was highly efficient and of high fidelity.
SDS-PAGE performed in this study confirmed that the constructed prokaryotic expression system pET32a-ltB-ureB-E. coli BL21DE3 could produce rLTB-UreB with high efficiency even when the concentration of IPTG was as low as 0.1 mmol/L. The inclusion body as a major form of rLTB-UreB and higher output (35% of the total bacterial proteins) of the recombinant protein was beneficial to industrial production.
The results of Western blotting in this study demonstrated that the rLTB-UreB could combine with both the commercial antibody against whole cell of H pylori and rabbit anti-rUreB serum. And this recombinant protein was able to efficiently induce rabbit to produce specific antibody. Furthermore, all the UreB antibody positive serum samples from 125 patients confirmed by our previous studies could recognize rLTB-UreB.
In the reports, the adjuvanticity of LTB was based on the binding ability to GM1 receptor on the surface of cell[27-33]. In this study, the strong binding to GM1 receptor of rLTB-UreB was confirmed by GM1-ELISA. Therefore, rLTB-UreB with qualified immunoreactivity, antigenicity and adjuvanticity could be used to develop H pylori genetically engineered vaccine at lower costs.
Edited by Zhu LH Proofread by Chen WW and Xu FM
| 1. | Zhang Z, Yuan Y, Gao H, Dong M, Wang L, Gong YH. Apoptosis, proliferation and p53 gene expression of H. pylori associated gastric epithelial lesions. World J Gastroenterol. 2001;7:779-782. [PubMed] |
| 2. | Michetti P, Kreiss C, Kotloff KL, Porta N, Blanco JL, Bachmann D, Herranz M, Saldinger PF, Corthésy-Theulaz I, Losonsky G. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in Helicobacter pylori-infected adults. Gastroenterology. 1999;116:804-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 231] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 3. | Suganuma M, Kurusu M, Okabe S, Sueoka N, Yoshida M, Wakatsuki Y, Fujiki H. Helicobacter pylori membrane protein 1: a new carcinogenic factor of Helicobacter pylori. Cancer Res. 2001;61:6356-6359. [PubMed] |
| 4. | Nakamura S, Matsumoto T, Suekane H, Takeshita M, Hizawa K, Kawasaki M, Yao T, Tsuneyoshi M, Iida M, Fujishima M. Predictive value of endoscopic ultrasonography for regression of gastric low grade and high grade MALT lymphomas after eradication of Helicobacter pylori. Gut. 2001;48:454-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 153] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3187] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 6. | Morgner A, Miehlke S, Fischbach W, Schmitt W, Müller-Hermelink H, Greiner A, Thiede C, Schetelig J, Neubauer A, Stolte M. Complete remission of primary high-grade B-cell gastric lymphoma after cure of Helicobacter pylori infection. J Clin Oncol. 2001;19:2041-2048. [PubMed] |
| 7. | Kate V, Ananthakrishnan N, Badrinath S. Effect of Helicobacter pylori eradication on the ulcer recurrence rate after simple closure of perforated duodenal ulcer: retrospective and prospective randomized controlled studies. Br J Surg. 2001;88:1054-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Yao YL, Zhang WD. Relation between Helicobacter pylori and gastric cancer. Shijie Huaren Xiaohua Zazhi. 2001;9:1045-1049. |
| 9. | Goto T, Nishizono A, Fujioka T, Ikewaki J, Mifune K, Nasu M. Local secretory immunoglobulin A and postimmunization gastritis correlate with protection against Helicobacter pylori infection after oral vaccination of mice. Infect Immun. 1999;67:2531-2539. [PubMed] |
| 10. | Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology. 1998;115:642-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 674] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 11. | Honda S, Fujioka T, Tokieda M, Satoh R, Nishizono A, Nasu M. Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer Res. 1998;58:4255-4259. [PubMed] |
| 12. | Hatzifoti C, Wren BW, Morrow WJ. Helicobacter pylori vaccine strategies--triggering a gut reaction. Immunol Today. 2000;21:615-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Corthésy-Theulaz I, Porta N, Glauser M, Saraga E, Vaney AC, Haas R, Kraehenbuhl JP, Blum AL, Michetti P. Oral immunization with Helicobacter pylori urease B subunit as a treatment against Helicobacter infection in mice. Gastroenterology. 1995;109:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 123] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Opazo P, Müller I, Rollán A, Valenzuela P, Yudelevich A, García-de la Guarda R, Urra S, Venegas A. Serological response to Helicobacter pylori recombinant antigens in Chilean infected patients with duodenal ulcer, non-ulcer dyspepsia and gastric cancer. APMIS. 1999;107:1069-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Suerbaum S, Josenhans C, Labigne A. Cloning and genetic characterization of the Helicobacter pylori and Helicobacter mustelae flaB flagellin genes and construction of H. pylori flaA- and flaB-negative mutants by electroporation-mediated allelic exchange. J Bacteriol. 1993;175:3278-3288. [PubMed] |
| 16. | Ghiara P, Rossi M, Marchetti M, Di Tommaso A, Vindigni C, Ciampolini F, Covacci A, Telford JL, De Magistris MT, Pizza M. Therapeutic intragastric vaccination against Helicobacter pylori in mice eradicates an otherwise chronic infection and confers protection against reinfection. Infect Immun. 1997;65:4996-5002. [PubMed] |
| 17. | Nilsson I, Utt M. Separation and surveys of proteins of Helicobacter pylori. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;771:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Marchetti M, Aricò B, Burroni D, Figura N, Rappuoli R, Ghiara P. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science. 1995;267:1655-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 409] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 19. | Rupnow MF, Owens DK, Shachter R, Parsonnet J. Helicobacter pylori vaccine development and use: a cost-effectiveness analysis using the Institute of Medicine Methodology. Helicobacter. 1999;4:272-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 20. | Pappo J, Thomas WD, Kabok Z, Taylor NS, Murphy JC, Fox JG. Effect of oral immunization with recombinant urease on murine Helicobacter felis gastritis. Infect Immun. 1995;63:1246-1252. [PubMed] |
| 21. | Tomb JF, White O, Kerlavage AR, Clayton RA, Sutton GG, Fleischmann RD, Ketchum KA, Klenk HP, Gill S, Dougherty BA. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2635] [Cited by in RCA: 2587] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 22. | Akada JK, Shirai M, Takeuchi H, Tsuda M, Nakazawa T. Identification of the urease operon in Helicobacter pylori and its control by mRNA decay in response to pH. Mol Microbiol. 2000;36:1071-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 122] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Labigne A, Cussac V, Courcoux P. Shuttle cloning and nucleotide sequences of Helicobacter pylori genes responsible for urease activity. J Bacteriol. 1991;173:1920-1931. [PubMed] |
| 24. | Verweij WR, de Haan L, Holtrop M, Agsteribbe E, Brands R, van Scharrenburg GJ, Wilschut J. Mucosal immunoadjuvant activity of recombinant Escherichia coli heat-labile enterotoxin and its B subunit: induction of systemic IgG and secretory IgA responses in mice by intranasal immunization with influenza virus surface antigen. Vaccine. 1998;16:2069-2076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Tochikubo K, Isaka M, Yasuda Y, Kozuka S, Matano K, Miura Y, Taniguchi T. Recombinant cholera toxin B subunit acts as an adjuvant for the mucosal and systemic responses of mice to mucosally co-administered bovine serum albumin. Vaccine. 1998;16:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Yamamoto M, McGhee JR, Hagiwara Y, Otake S, Kiyono H. Genetically manipulated bacterial toxin as a new generation mucosal adjuvant. Scand J Immunol. 2001;53:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | de Haan L, Feil IK, Verweij WR, Holtrop M, Hol WG, Agsteribbe E, Wilschut J. Mutational analysis of the role of ADP-ribosylation activity and GM1-binding activity in the adjuvant properties of the Escherichia coli heat-labile enterotoxin towards intranasally administered keyhole limpet hemocyanin. Eur J Immunol. 1998;28:1243-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Saito K, Shoji J, Inada N, Iwasaki Y, Sawa M. Immunosuppressive effect of cholera toxin B on allergic conjunctivitis model in guinea pig. Jpn J Ophthalmol. 2001;45:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Tamura S, Hatori E, Tsuruhara T, Aizawa C, Kurata T. Suppression of delayed-type hypersensitivity and IgE antibody responses to ovalbumin by intranasal administration of Escherichia coli heat-labile enterotoxin B subunit-conjugated ovalbumin. Vaccine. 1997;15:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Douce G, Fontana M, Pizza M, Rappuoli R, Dougan G. Intranasal immunogenicity and adjuvanticity of site-directed mutant derivatives of cholera toxin. Infect Immun. 1997;65:2821-2828. [PubMed] |
| 31. | Xia XP, Yan J, Zhao SF. [Cloning, expression and identification of Escherichia coli LTB gene and Vibrio cholerae CTB gene]. Zhejiang Daxue Xuebao Yixueban. 2003;32:17-20. [PubMed] |
| 32. | Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning, A Laboratory Manual [M]. 2nd edition. New York:. Cold Spring Harbor Laboratory Press. 1989;pp1.21-1.52, 2.60-2.80, 7.3-7.35, 9.14-9.22. |
| 33. | de Haan L, Holtrop M, Verweij WR, Agsteribbe E, Wilschut J. Mucosal immunogenicity of the Escherichia coli heat-labile enterotoxin: role of the A subunit. Vaccine. 1996;14:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |