Published online Sep 1, 2004. doi: 10.3748/wjg.v10.i17.2609
Revised: March 1, 2004
Accepted: March 4, 2004
Published online: September 1, 2004
Cystic dystrophy of the duodenal wall is a rare condition characterized by the development of cysts in heterotopic pancreatic tissue localized in the duodenal wall. A 38-year-old man was admitted to the hospital for abdominal pain and vomiting after food intake. The diagnosis of acute pancreatitis was initially suspected. Abdominal ultrasound examination revealed thickening of the second portion of duodenal wall within which, small cysts (diameter, less than 1 cm) were present in the vicinity of pancreatic head. The head of pancreas appeared enlarged (63 mm × 42 mm) and hypoechoic. Upper endoscopy and barium X-ray series were performed revealing a severe circumferential deformation, as well as 4 cm long stenosis of the second portion of the duodenum. CT examination revealed multiple cysts located in an enlarged, thickened duodenal wall with moderate to strong post-contrast enhancement. We suspected that patient had cystic dystrophy of duodenal wall developed in the heterotopic pancreas and diagnosis was confirmed by endoscopic ultrasound (EUS). Endoscopic ultrasound (EUS) revealed circular stenosis from the duodenal bulb onwards. A twenty megaHertz mini-probe examination further showed diffuse (intramural) infiltration of duodenal wall limited to the submucosa and muscularis propria of the second portion of duodenum with multiple microcysts within the thickened mucosa and submucosa. Patient was successfully surgically treated and pancreatoduodenectomy was performed. The pathological examination confirmed a diagnosis of cystic dystrophy of a heterotopic pancreas. Endoscopic ultrasonography features allow preoperative diagnosis of cystic dystrophy of a heterotopic pancreas in duodenal wall, with intraluminal 20 MHz mini probe sonography being more efficient in cases of luminal stenosis.
- Citation: Jovanovic I, Knezevic S, Micev M, Krstic M. EUS mini probes in diagnosis of cystic dystrophy of duodenal wall in heterotopic pancreas: A case report. World J Gastroenterol 2004; 10(17): 2609-2612
- URL: https://www.wjgnet.com/1007-9327/full/v10/i17/2609.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i17.2609
The most common type of heterotopic tissue in the gastrointestinal tract is pancreas. Pancreatic heterotopia is defined as the presence of abnormally located (aberrant) pancreatic tissue with no contact (vascular, neural, and anatomical) with the normal pancreas, and possesses its own duct system and vascular supply[1,2]. Heterotopic pancreatic tissue has been found in several abdominal and intrathoracic locations, most frequently in the stomach (25%-60%) or duodenum (25%-35%)[3].
Cystic dystrophy of the duodenal wall in heterotopic pancreas is a relatively rare clinical disorder affecting young men in particular[4]. Diagnosis is usually suspected during initial investigation of non-specific clinical symptoms such as abdominal pain, duodenal obstruction and weight loss and finally confirmed by imaging techniques, mainly endoscopic ultrasound which localizes precisely cysts in duodenal wall.
The purpose of this report was to present a case with this anomaly and provide up-to-date literature review on this topic.
A 38-year-old man who was admitted for clarification of several months of upper abdominal pain, vomiting after food intake accompanied by a weight loss. The patient had no history of alcohol abuse, and smoked 20 cigarettes per day. Physical examination revealed tachypnea and tachycardia, and a blood pressure 100/70 mmHg. There was abdominal tenderness in upper abdomen. Hematological and blood bio-chemical tests were performed (Tables 1, Tables 2) showing a sevenfold increased level of serum amylase (625 U/L, range 20-90 U/L) accompanied by an elevated alkaline phosphatase, and markers of acute hepatocyte injury such as alanine (AST) and aspartate aminotransferase (ALT) and gama GT. Bilirubin levels remained normal. The urine had a pH6.5 and a specific gravity of 1.010. The sediment contained 3-5 red cells and 0-2 white cells per high-power field. A diagnosis of acute pancreatitis was initially suspected.
| Variable | Value | Initial tests | Repeated stests |
| Hematocrit (%) | 35-54 | 43 | 49.7 |
| White cell count (× 109) | 4.0-10.0 | 12.0 | 17.7 |
| Differential count (× 109) | |||
| Neutrophils | 1.4-6.5 | 4.3 | 2.8 |
| Lymphocytes | 1.2-3.4 | 5.6 | 2.8 |
| Monocytes | 0.1-0.6 | 2.1 | 1.1 |
| Prothrombin time (% quick) | 75-120 | 114 | 115 |
| Variable | Initial test | Repeated test |
| Sodium (mmol/L) | 132 | 148 |
| Potassium (mmol/L) | 2.5 | 4.6 |
| Chloride (mmol/L) | 75 | 107 |
| Urea nitrogen (mmol/L) | 9.4 | 2.8 |
| Creatinine (μmol/L) | 80 | 57 |
| Glucose (mmol/L) | 8.5 | 4.3 |
| Bilirubin (μmol/L) | 13 | 13 |
| AST (U/L) | 50 | 153 |
| LT (U/L) | 93 | 217 |
| Alkaline phosphatase (U/L) | 361 | 980 |
| Alpha amylase (U/L) | 627 | 354 |
Ultrasonographic examination revealed an enlarged steatotic liver, no stones in gall bladder, and a common bile duct diameter of 8 mm with no signs of stone obstruction. Second portion duodenal wall appeared thicker within which, small cysts (diameter, less than 1 cm) were present in the vicinity of pancreatic head. The head of pancreas appeared enlarged (63 mm × 42 mm) and hypoechoic.
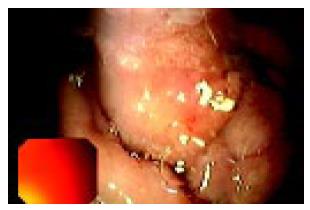
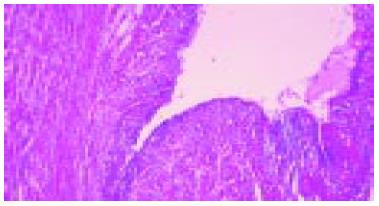
An upper GI endoscopy also revealed a severe semispherical deformation of second portion of duodenum without mucosal lesions. Due to the stenosis the endoscope could not be passed distal to upper duodenal flexure. Biopsy of the duodenal mucosa detected moderate inflammatory changes (Figure 1).
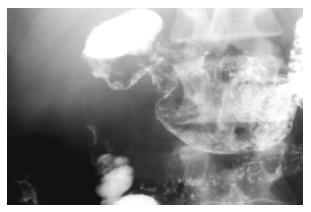
A barium meal revealed a severe circumferential deformation, as well as 4 cm long stenosis of the second portion of the duodenum (Figure 2).
We suspected that the patient had cystic dystrophy of duodenal wall developed in the heterotopic pancreas which was highly indicated by computed tomography and endoscopic ultrasound (EUS).
CT examinations revealed multiple cysts located in an enlarged, thickened duodenal wall with moderate to strong post-contrast enhancement.
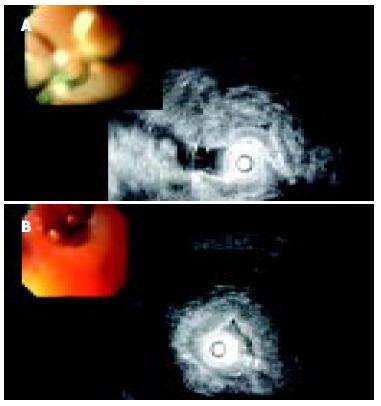
Endoscopic ultrasound (EUS) revealed circular stenosis from the duodenal bulb onwards. Twenty megaHertz mini-probe examinations further showed diffuse (intramural) infiltration of duodenal wall limited to the submucosa and muscularis propria of the second portion of duodenum with multiple, very small anechoic spots, like microcysts (Figure 3A, B). EUS did not reveal any tumors of the pancreas.
MRI examinations showed a suspicious 6 cm × 4 cm solid and partly cystic tumor of processus uncinatus of pancreas.
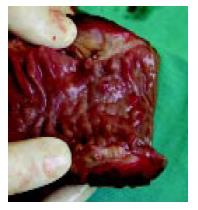
The patient was successfully surgically treated and pancreatoduodenectomy was performed (Figure 4). Pathological examinations confirmed a diagnosis of cystic dystrophy of a heterotopic pancreas (Figure 5).
The pancreas develops from two primitive diverticula, dorsal and ventral, that arise from the duodenum and the base of the liver, respectively, in the fifth week of gestation. During the seventh week, the two primordia fuse. The ventral portion gives rise to part of the head of the pancreas and the uncinate process, and the dorsal portion becomes the body. In more than half the cases of pancreatic heterotopia, pancreatic tissue has been located in the duodenum or the pylorus but other sites have also been involved[2].
Pancreatic heterotopia can develop due to either metaplasia of multipotent endodermal cells in situ or transplantation of embryonic pancreatic cells to adjacent structures[2]. Heterotopic pancreatic tissues may be found in the mucosa or the muscularis or may be attached to the serosa of the gastrointestinal tract. Heterotopic pancreatic tissues that lack both acinar and endocrine cells have been called myoepithelial hamartoma, adenomyosis, or adenomyoma. Despite its congenital origin, pancreatic heterotopia was usually discovered in adults due to its complications[5]. Most frequently it was found incidentally during autopsies, operations or during endoscopic examinations of the upper GI tract[6]. The occurrence rate of heterotopic pancreas in autopsy series was estimated to be about 0.55% to 13.7%[4,7].
Despite many advances of new diagnostic tools and methods, the diagnosis of heterotopic pancreas prior to surgery is still difficult. Heterotopic pancreas is usually asymptomatic and does not require treatment (i.e. surgical excision). However, sometimes, there were evident clinical symptoms[8]. The most common symptoms attributed to heterotopic pancreas were: abdominal pain, nausea, vomiting, anemia, weight loss and melena[9]. Rare manifestations and complications of heterotopic pancreas included acute and chronic pancreatitis[10], biliary obstruction, intestinal obstruction, cystic dystrophy and malignant degeneration[5,11,12].
The differential diagnosis of a cystic dystrophy of heterotopic pancreas includes disorders in three broad categories: inflammatory lesions, neoplasms, and congenital anomalies.
Complications of pancreatic heterotopia usually include inflammation with a formation of an inflammatory mass, ulceration, bleeding and obstruction with clinical manifestations of acute and chronic pancreatitis. Chronic pancreatitis with mucinous metaplasia of the ductal epithelium can further be complicated by prominent exudation of mucin into the stroma, mimicking mucinous carcinoma[13].
The most challenging differential diagnosis of cystic dystrophy of heterotopic pancreas is cystic (mucinous) neoplasms. It has been demonstrated that pancreatic carcinomas or endocrine tumors can also develop in heterotopic pancreatic tissue[14,15]. In this case, CT findings are non-specific. Endoscopic and upper GI barium X-ray series can reveal duodenal stenosis and submucosal masses if there are no mucosal lesions and biopsies from the lesions cannot always provide a representative tissue specimen. The introduction of endoscopic ultrasonography (EUS) makes the diagnosis of cystic dystrophy of the duodenal wall in heterotopic pancreas easier[16]. Furthermore, endoscopic ultrasonography is a reliable method providing precise localization, extent and characteristics of the submucosal mass, making possible to differentiate it from all other causes of stenosis such as tumor and pancreas annulare[17].
However, although there is good correlation between EUS[16], computer tomography (CT) and nuclear magnetic resonance (NMR) findings with histological changes[17,18], the definite diagnosis is often made only from surgical excision.
The treatment of this disease is controversial. There are reports of successful treatment with long-acting somatostatin synthetic stable analog[19]. In some 40% of patients reduction in cyst size could be accomplished after three months of treatment[16]. But, on the other hand, octreotide/analog treatment was of a limited value regarding already formed stenosis of the duodenum (in regards to persistent duodenal stenosis)[20,21]. Furthermore, the necessary duration of treatment has not been established yet.
Ponchon et al[22] offered an alternative to surgical treatment for endoscopic fenestration of cysts, but that approach was feasible only for cases where cysts were fewer, not dispersed and relatively larger in size.
Despite the encouraging attempts to treat cystic dystrophy of duodenal wall in heterotopic pancreas with conservative approaches, the treatment is still primarily surgical[20,21,23,24].
Moreover, there is a difficult therapeutic dilemma: if such a lesion should be treated by duodenopancreatectomy or limited local excision?
Regarding the surgical procedures, there were data that conservative surgical procedures including segmental duodenal resection could be an alternative approach to the Whipple procedure[25], but in other cases of pancreatic surgery, they were associated with a considerable morbidity and mortality[26-28], and should be reserved for specialized centers[29].
In conclusion, we demonstrated a rare case of cystic dystrophy of heterotopic pancreas in duodenal wall. Cystic dystrophy of aberrant pancreatic tissue can be either isolated or as in our case associated with acute pancreatitis and duodenal stenosis. Although the condition is benign, clinical symptoms vary and patients are usually referred for suspected pancreatic neoplasms or acute pancreatitis. Endoscopic ultrasonographic features allow preoperative diagnosis of cystic dystrophy of a heterotopic pancreas in duodenal wall, with intraluminal 20 MHz mini-probe sonography being more efficient in cases of luminal stenosis.
Edited by Zhu LH Proofread by Xu FM
| 1. | Dolan RV, ReMine WH, Dockerty MB. The fate of heterotopic pancreatic tissue. A study of 212 cases. Arch Surg. 1974;109:762-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 213] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Skandalakis JE, Grey SW. Embriology for surgeons: the embryological basis for treatment of congenital anomalies. 2nd editor. Baltimore: Williams Wilkins 1994; 366-387. |
| 3. | Moen J, Mack E. Small-bowel obstruction caused by heterotopic pancreas in an adult. Am Surg. 1989;55:503-504. [PubMed] |
| 5. | Burke GW, Binder SC, Barron AM, Dratch PL, Umlas J. Heterotopic pancreas: gastric outlet obstruction secondary to pancreatitis and pancreatic pseudocyst. Am J Gastroenterol. 1989;84:52-55. [PubMed] |
| 6. | Jaffe R. The pancreas. Textbook of Fetal and Perinatal Pathology. Vol 2. Boston, Mass: Blackwell Scientific 1991; 1021-1055. |
| 7. | Armstrong CP, King PM, Dixon JM, Macleod IB. The clinical significance of heterotopic pancreas in the gastrointestinal tract. Br J Surg. 1981;68:384-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 130] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Pang LC. Pancreatic heterotopia: a reappraisal and clinicopathologic analysis of 32 cases. South Med J. 1988;81:1264-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 55] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Hsia CY, Wu CW, Lui WY. Heterotopic pancreas: a difficult diagnosis. J Clin Gastroenterol. 1999;28:144-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Chung JP, Lee SI, Kim KW, Chi HS, Jeong HJ, Moon YM, Kang JK, Park IS. Duodenal ectopic pancreas complicated by chronic pancreatitis and pseudocyst formation--a case report. J Korean Med Sci. 1994;9:351-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Fléjou JF, Potet F, Molas G, Bernades P, Amouyal P, Fékété F. Cystic dystrophy of the gastric and duodenal wall developing in heterotopic pancreas: an unrecognised entity. Gut. 1993;34:343-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 97] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Jeng KS, Yang KC, Kuo SH. Malignant degeneration of heterotopic pancreas. Gastrointest Endosc. 1991;37:196-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Nopajaroonsri C. Mucus retention in heterotopic pancreas of the gastric antrum. A lesion mimicking mucinous carcinoma. Am J Surg Pathol. 1994;18:953-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Kaneda M, Yano T, Yamamoto T, Suzuki T, Fujimori K, Itoh H, Mizumoto R. Ectopic pancreas in the stomach presenting as an inflammatory abdominal mass. Am J Gastroenterol. 1989;84:663-666. [PubMed] |
| 15. | Al Jitawi SA, Hiarat AM, Al-Majali SH. Diffuse myoepithelial hamartoma of the duodenum associated with adenocarcinoma. Clin Oncol. 1984;10:289-293. [PubMed] |
| 16. | Palazzo L, Borotta E, Napoleon B. Is endoscopic ultrasonography accurate for the localization of pancreatic and duodenal tumors in patients with multiple endocrine neoplasia type I. Gastroenterology. 1994;106:A313. |
| 17. | Vullierme MP, Vilgrain V, Fléjou JF, Zins M, O'Toole D, Ruszniewski P, Belghiti J, Menu Y. Cystic dystrophy of the duodenal wall in the heterotopic pancreas: radiopathological correlations. J Comput Assist Tomogr. 2000;24:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Procacci C, Graziani R, Zamboni G, Cavallini G, Pederzoli P, Guarise A, Bogina G, Biasiutti C, Carbognin G, Bergamo-Andreis IA. Cystic dystrophy of the duodenal wall: radiologic findings. Radiology. 1997;205:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Basili E, Allemand I, Ville E, Laugier R. [Lanreotide acetate may cure cystic dystrophy in heterotopic pancreas of the duodenal wall]. Gastroenterol Clin Biol. 2001;25:1108-1111. [PubMed] |
| 20. | Rubay R, Bonnet D, Gohy P, Laka A, Deltour D. Cystic dystrophy in heterotopic pancreas of the duodenal wall: medical and surgical treatment. Acta Chir Belg. 1999;99:87-91. [PubMed] |
| 21. | Bittar I, Cohen Solal JL, Cabanis P, Hagege H. [Cystic dystrophy of an aberrant pancreas. Surgery after failure of medical therapy]. Presse Med. 2000;29:1118-1120. [PubMed] |
| 22. | Ponchon T, Napoleon B, Hedelius F, Bory R. Traitement endoscopique de la dystrophie kistique de la paroi duodenale. Gastroenterol Clin Biol. 1997;21:A63. |
| 23. | Glaser M, Roskar Z, Skalicky M, Krajnc I. Cystic dystrophy of the duodenal wall in a heterotopic pancreas. Wien Klin Wochenschr. 2002;114:1013-1016. [PubMed] |
| 24. | Wind P, Pardies P, Roullet MH, Rouzier R, Zinzindohoué F, Cugnenc PH. [Cystic dystrophy of the duodenal wall in aberrant pancreas]. Ann Chir. 1999;53:164-167. [PubMed] |
| 25. | Marmorale A, Tercier S, Peroux JL, Monticelli I, Mc Namara M, Huguet C. [Cystic dystrophy in heterotopic pancreas of the second part of the duodenum. One case of conservative surgical procedure]. Ann Chir. 2003;128:180-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Lansing PB, Blalock JB, Ochsner JL. Pancreatoduodenectomy: a retrospective review 1949 to 1969. Am Surg. 1972;38:79-86. [PubMed] |
| 27. | Connolly MM, Dawson PJ, Michelassi F, Moossa AR, Lowenstein F. Survival in 1001 patients with carcinoma of the pancreas. Ann Surg. 1987;206:366-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 184] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Herter FP, Cooperman AM, Ahlborn TN, Antinori C. Surgical experience with pancreatic and periampullary cancer. Ann Surg. 1982;195:274-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 125] [Article Influence: 2.9] [Reference Citation Analysis (0)] |