Published online Sep 1, 2004. doi: 10.3748/wjg.v10.i17.2602
Revised: February 4, 2004
Accepted: February 24, 2004
Published online: September 1, 2004
A 59-year-old woman was admitted to our hospital because of recurrent follicular lymphoma (FL). Colonoscopic examination revealed a rectal submucosal tumor (SMT) without any erosions and ulcers. In this patient, it was difficult to distinguish non-Hodgkin’s lymphoma (NHL) invasion from other disorders of the colon including carcinoid tumor merely based on endoscopic findings. Histopathologic and immunohistochemical studies on biopsy specimens showed an infiltration of atypical lymphocytes that were positive for CD20 and BCL2 but negative for UCHL-1. Fluorescence in situ hybridization on paraffin-embedded tissue sections (T-FISH) identified a translocation of BCL2 with IGH gene. Based on these findings, the tumor was defined as an invasion of FL. T-FISH method is useful for the detection of a monoclonality of atypical lymphocytes in an SMT of the gastrointestinal tract, and particularly for the detection of chromosomal translocations specific to lymphoma subtypes.
-
Citation: Yoshida N, Nomura K, Matsumoto Y, Nishida K, Wakabayashi N, Konishi H, Mitsufuji S, Kataoka K, Okanoue T, Taniwaki M. Detection of
BCL2-IGH rearrangement on paraffin-embedded tissue sections obtained from a small submucosal tumor of the rectum in a patient with recurrent follicular lymphoma. World J Gastroenterol 2004; 10(17): 2602-2604 - URL: https://www.wjgnet.com/1007-9327/full/v10/i17/2602.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i17.2602
Primary gastrointestinal (GI) lymphomas are uncommon tumors, constituting less than 2% of all GI malignancies[1]. The stomach, however, is the most frequent site of extranodal NHL. Of all GI NHLs, the incidence of gastric lymphomas ranged from 51% to 86%[2,3], while that of colonic lymphomas ranged from 1.6% to 16.2%[4,5]. Diagnosis of GI lymphomas may be complicated due to difficulties in pathological diagnosis and in staging with endoscopic biopsies. In fact, it is occasionally difficult not only to distinguish NHL from other tumors but also to define subtypes of NHL on the basis of endoscopic and histological findings of biopsy specimens[6,7]. Although flowcytometric analysis is efficient for differential diagnosis of lymphomas, obtaining a diagnostic biopsy may sometimes be difficult because GI lymphomas spread submucosally and have normal surface qualities; therefore, a number of specimens are required for an accurate diagnosis.
On the other hand, chromosomal translocations are closely associated with distinctive subtypes of malignant lymphoma[8]. Both chromosomal banding and fluorescence in situ hybridization (FISH) are used for the detection of specific translocation. In addition, we have developed a procedure using FISH directly on paraffin embedded tissue sections (T-FISH), enabling us to perform cytogenetic analyses on both archival and small biopsy specimens[9,10].
In the current study, using T-FISH we detected rearrangement of the BCL2 with immunoglobulin heavy chain (IGH) gene on biopsy specimens obtained from a small rectal submucosal tumor (SMT), thereby differentiating follicular lymphoma (FL) from other neoplastic tumors of colon as well as other types of lymphoma.
A 59-year-old woman was admitted to our hospital because of the swelling of multiple cervical lymph nodes. The patient had suffered from stage IV disease of FL since 1997. Although multiple chemotherapies including high dose regimen supported by autologous hematopoietic stem cell transplantation were administered, complete remission has not been achieved. At the current hospitalization, the patient showed elevated soluble interleukin-2 receptor (1024 U/L), while no abnormal cells were detected in both the peripheral blood and bone marrow. Gallium-scintigraphy showed abnormal accumulation at cervical and supra-clavicular lesions. Gastroscopic examination revealed no remarkable abnormalities, whereas colonoscopic examination detected a small elevated lesion (7 mm × 8 mm in size) on the rectum.
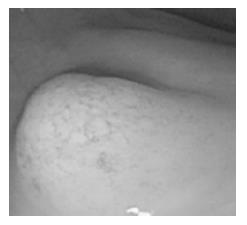
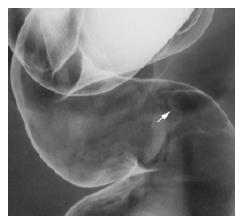
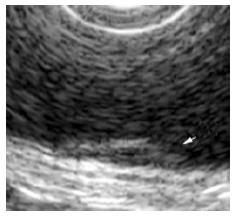
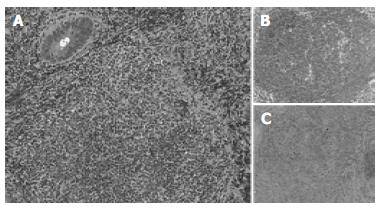
The surface of tumor was slightly reddish, but no erosions and ulcers were noted (Figure 1). No definitive deformity of colonic wall was detected with double-contrast barium enema (Figure 2). The tumor invasion was confined to the second layer of colonic wall based on the endoscopic ultrasonographic findings (Figure 3). Flowcytometric analyses were not able to be performed because of small specimens obtained from diagnostic biopsies. Histopathologic and immunohistochemical studies on biopsy specimens showed the aggregation of atypical medium-sized lymphocytes having irregularly enlarged nuclei that were positive for CD20, CD10 and BCL2, but negative for UCHL-1 and CD5 (Figures 4A-C).
Using DHAP regimen (cisplatin 70 mg/m2, Ara-C 1.4 g/m2× 2, dexamethasone 40 mg × 4), the patient achieved a partial response, followed by the administration of rituximab. The rectal lesion disappeared on d 120.
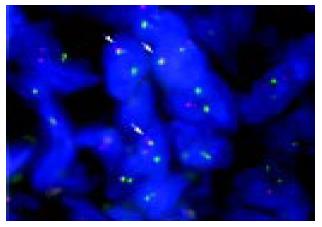
In order to confirm a diagnosis of lymphoma and define specific chromosomal abnormalities, we performed T-FISH analyses on the sample obtained from a rectal SMT according to the protocol already described elsewhere[9,10]. Briefly, sections from paraffin-embedded tissues were placed on slides, and then deparaffinized in xylene. Each slide was dehydrated in ethanol and treated with 0.2 mol/L HCl and 0.05 mg/mL proteinase K in TEN (0.05 mol/L tris-HCl, pH7.8, 0.01 mol/L EDTA, and 0.01 mol/L NaCl buffer) for 10 min at 37 °C. FISH probes and samples were denatured simultaneously for 10 min at 90 °C, and were hybridized overnight at 42 °C. LSI IGH/BCL2, IGH/BCL1 and MALT1 probes (Vysis, CA, USA) were used for the detection of t (14; 18), t (11; 14), and 18q21 translocations, respectively. Images of signal were captured by a CCD camera. T-FISH detected fusion signals of BCL2 and IGH genes on 75 of 100 nuclei (Figure 5), defining the invasion of FL with t (14; 18) (q32; q21). On the other hand, 95 of 100 nuclei showed negative results for both t (11; 14) and 18q21 translocations.
We demonstrated rearrangement of BCL2 with IGH gene on a small rectal SMT in a patient with recurrent lymphoma using T-FISH, thereby defining colonic involvement of FL. It is difficult to discriminate NHL from other disorders of the GI tract only based on endoscopic and histological findings[11]. Although endoscopic procedures are important not only in the detection of other disorders involving GI tract, but also in providing a means for pathologic diagnosis through biopsy, it should be noted, however, that obtaining a diagnostic biopsy may be difficult because GI lymphomas spread submucosally and have normal surface qualities. Since most GI lymphomas and other malignancies including adenocarcinoma and carcinoid tumor frequently display a polypoid growth pattern involving the mucosa, submucosa and muscularis[12], it is sometimes difficult to make differential diagnosis based on the histological findings.
In the current patient, immunohistochemical studies defined a diagnosis of lymphoid lesion of a rectal SMT, although flowcytometric analysis was not successful because of small specimens obtained from diagnostic biopsy. There are many kinds of SMTs originating in the colon, for example, lipoma, malignant lymphoma, carcinoid tumor, etc[13]. Flowcytometric and immunocytochemical studies are useful for demonstration of a monoclonal population of lymphocytes of a lesion of interest, since most GI lymphomas are virtually always of the B-cell type. However, it should be noted that there are certain subtypes preferentially involving GI tract including mantle cell lymphoma, and mucosa-associated lymphoid tissue (MALT) lymphoma[14]. In this respect, cytogenetic study is necessary for the determination of these subtypes, since chromosomal translocations are closely associated with distinctive subtypes of NHL[14]. More than 90% of mantle cell lymphoma was related to the translocation of IGH with BCL1 gene[15]. MALT lymphoma was related to the translocation of API2 with MALT1 at frequencies of 18.8%[9]. Approximately 60% of FL showed the translocation of BCL2 with IGH (Matsumoto et al in press). Hence, T-FISH is a useful method not only to make definitive diagnosis of NHL but also to define its subtypes. In addition, a few biopsy samples are enough for T-FISH analysis when compared with flowcytometric analysis and polymerase chain reaction (PCR). Furthermore, the tissues embedded for a period of 15 years were available for T-FISH analysis[9,10].
At present there is considerable controversy concerning the treatment of primary and secondary GI lymphomas. Rituximab has recently included in treatment option[16]. In our patient with recurrent FL, salvage therapy including high-dose Ara-C with rituximab was performed. Because API2-MALT1-positive lymphoma has demonstrated a more aggressive subgroup[17], T-FISH will provide novel information for the selection of treatment for GI lymphomas.
In conclusion, our results indicate that T-FISH is a promising procedure for the routine detection of genetic alterations in GI lymphomas, it enables us to not only make definitive diagnosis of NHL but also to define its subtypes.
Edited by Zhu LH Proofread by Xu FM
| 1. | Frazee RC, Roberts J. Gastric lymphoma treatment. Medical versus surgical. Surg Clin North Am. 1992;72:423-431. [PubMed] |
| 2. | Radaszkiewicz T, Dragosics B, Bauer P. Gastrointestinal malignant lymphomas of the mucosa-associated lymphoid tissue: factors relevant to prognosis. Gastroenterology. 1992;102:1628-1638. [PubMed] |
| 3. | Hansen PB, Vogt KC, Skov RL, Pedersen-Bjergaard U, Jacobsen M, Ralfkiaer E. Primary gastrointestinal non-Hodgkin's lymphoma in adults: a population-based clinical and histopathologic study. J Intern Med. 1998;244:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Otter R, Bieger R, Kluin PM, Hermans J, Willemze R. Primary gastrointestinal non-Hodgkin's lymphoma in a population-based registry. Br J Cancer. 1989;60:745-750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Crump M, Gospodarowicz M, Shepherd FA. Lymphoma of the gastrointestinal tract. Semin Oncol. 1999;26:324-337. [PubMed] |
| 6. | Takenaga T, Yamamoto N, Yamanaka A, Yamada M, Takasimizu K, Sasabe M, Tamura Y, Kurosawa K, Fujimoto H, Ookusa T. Gastrointestinal involvement of malignant lymphoma. Gastroenterol Endosc. 1988;30:2218-2224. |
| 7. | Damaj G, Verkarre V, Delmer A, Solal-Celigny P, Yakoub-Agha I, Cellier C, Maurschhauser F, Bouabdallah R, Leblond V, Lefrère F. Primary follicular lymphoma of the gastrointestinal tract: a study of 25 cases and a literature review. Ann Oncol. 2003;14:623-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Ong ST, Le Beau MM. Chromosomal abnormalities and molecular genetics of non-Hodgkin's lymphoma. Semin Oncol. 1998;25:447-460. [PubMed] |
| 9. | Nomura K, Yoshino T, Nakamura S, Akano Y, Tagawa H, Nishida K, Seto M, Nakamura S, Ueda R, Yamagishi H. Detection of t (11; 18) (q21; q21) in marginal zone lymphoma of mucosa-associated lymphocytic tissue type on paraffin-embedded tissue sections by using fluorescence in situ hybridization. Cancer Genet Cytogenet. 2003;140:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Nomura K, Sekoguchi S, Ueda K, Nakao M, Akano Y, Fujita Y, Yamashita Y, Horiike S, Nishida K, Nakamura S. Differentiation of follicular from mucosa-associated lymphoid tissue lymphoma by detection of t (14; 18) on single-cell preparations and paraffin-embedded sections. Genes Chromosomes Cancer. 2002;33:213-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Shepherd NA, Hall PA, Coates PJ, Levison DA. Primary malignant lymphoma of the colon and rectum. A histopathological and immunohistochemical analysis of 45 cases with clinicopathological correlations. Histopathology. 1988;12:235-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 124] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Coulson WF. The Stomach. Surgical Pathology, Philadelphia: J.B. Lippincott Company 1988; 123-125. |
| 13. | Kawamoto K, Yamada Y, Furukawa N, Utsunomiya T, Haraguchi Y, Mizuguchi M, Oiwa T, Takano H, Masuda K. Endoscopic submucosal tumorectomy for gastrointestinal submucosal tumors restricted to the submucosa: a new form of endoscopic minimal surgery. Gastrointest Endosc. 1997;46:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Jaffe ES, Harris NL, Stein H, Vardiman JW, editors . Tumours of Haematopoietic and Lymphoid Tissues, 3rd editor. Lyon France: IARC 2001; . |
| 15. | Hui P, Howe JG, Crouch J, Nimmakayalu M, Qumsiyeh MB, Tallini G, Flynn SD, Smith BR. Real-time quantitative RT-PCR of cyclin D1 mRNA in mantle cell lymphoma: comparison with FISH and immunohistochemistry. Leuk Lymphoma. 2003;44:1385-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Press OW, Unger JM, Braziel RM, Maloney DG, Miller TP, LeBlanc M, Gaynor ER, Rivkin SE, Fisher RI. A phase 2 trial of CHOP chemotherapy followed by tositumomab/iodine I 131 tositumomab for previously untreated follicular non-Hodgkin lymphoma: Southwest Oncology Group Protocol S9911. Blood. 2003;102:1606-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 114] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Sakugawa ST, Yoshino T, Nakamura S, Inagaki H, Sadahira Y, Nakamine H, Okabe M, Ichimura K, Tanimoto M, Akagi T. API2-MALT1 fusion gene in colorectal lymphoma. Mod Pathol. 2003;16:1232-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |