Published online Sep 1, 2004. doi: 10.3748/wjg.v10.i17.2595
Revised: December 4, 2003
Accepted: December 16, 2003
Published online: September 1, 2004
AIM: To investigate the effects of dendritic cells (DCs) transfected with full-length wild-type p53 and stimulated by gastric cancer lysates on immune response.
METHODS: The wild-type p53 was transduced to DCs with adenovirus, and the DCs were stimulated by gastric cancer lysates. The surface molecules (B7-1, B7-2, MHC-I, MHC-II) of all DCs were detected by FACS, and the ability of the DCs to induce efficient and specific immunological response in anti-51Cr-labeled target cells was studied. BALB/c mice injected with DCs and Mk28 were established, and CTL response in mice immunized with Lywt-p53DC was evaluated. Tumor-bearing mice were treated with Lywt-p53DC.
RESULTS: The surface molecules of Lywt-p53DC had a high expression of B7-1 (86.70% ± 0.07%), B7-2 (18.77% ± 0.08%), MHC-I (87.20% ± 0.05%) and MHC-II (56.70% ± 0.07%); T lymphocytes had a specific CTL lysis ability induced by Lywt-p53DC; the CTL lysis rate was as high as 81%. The immune protection of Lywtp-53DC was obvious, the tumor diameter in Lywtp-53DC group was 3.10 ± 0.31 mm, 2.73 ± 0.23 mm, 3.70 ± 0.07 mm on d 13, 16 and 19, respectively, which were smaller than control, DC, wtp53DC and LyDC group (P < 0.05). Tumor growth rate in Lywtp53DC group was slower than that in other groups (P < 0.05).
CONCLUSION: DCs transfected with wild-type p53 and stimulated by gastric cancer lysates have specific CTL killing activity.
-
Citation: Sun HW, Tang QB, Cheng YJ, Zou SQ. Effects of dendritic cells transfected with full-length wild-type
p53 and stimulated by gastric cancer lysates on immune response. World J Gastroenterol 2004; 10(17): 2595-2597 - URL: https://www.wjgnet.com/1007-9327/full/v10/i17/2595.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i17.2595
Dendritic cells (DCs) are the most potent antigen-presenting cells and are actively used in cancer immunotherapy. The wild-type p53 can be recognized as an antigen and can induce specific CTLs in the host body. It is an effective method to immunize body with p53 in p53-overexpressing tumor cells. p53-based immunization is an attractive approach to cancer immunotherapy due to the accumulation of p53 protein in gastric cancer. We detected the effects of DCs transfected with full-length wild-type p53 and modified by gastric cancer lysates on immune response and tried to make DC induce efficient and specific anti-tumor immunological response.
Eight-week-old female BALB/c mice were purchased from Hubei Animal Center and housed in pathogen-free units of Tongji Hospital experiment center.
Gastric cancer cells (Mk28) were obtained from Hubei cell culture center and cultured in complete culture medium (CCM) containing RPMI 1640 supplemented with 25 mL HEPES, 100 mL/LFCS, and antibiotics (100 U/mL penicillin, 100 μg/mL streptomycin, 0.25 μg/mL amphotericin B). It is a relatively immunogenic tumor that carries a mutant endogenous p53 gene. P815 mastocytoma cells were obtained from ATC Company and cultured in CCM. The cell cultures were maintained at 37 °C in 50 mL/L CO2 humidified atmosphere.
Ad-mp53 was constructed by cloning the 1.5-kb murine p53 cDNA (obtained from Sigma, Wuhan, China) into pAd1/CMV that contains CMV promoter and bovine growth hormone polyA signal sequence. This plasmid was co-transfected with pBHG10 into 293 cells. Recombinant adenovirus was selected based on PCR analysis of individual plaques. Control adenovirus (Ad-c) was prepared by deletion of E1 region from adenovirus serotype.
FITC-labeled mouse anti-mouse p53 antibody and isotype mouse IgG2a were purchased from Serotec Company (New York, USA). FITC-labeled anti-mouse CD11c antibody and isotype mouse IgG2a, k, PE-labeled anti-mouse I-Ad, anti-mouse B7-1, B7-2, MHC-I, MHC-II antibody and isotype mouse IgG2a, k, as well as hamster anti-mouse CD40 (HM40-3) monoclonal antibody and anti-hamster IgM were purchased from Sigma Company.
DCs were generated from bone marrow of naive syngeneic mice in CCM supplemented with 20 ng/mL murine GM-CSF, 10 ng/mL IL-4, and 50 μmol/L 2-mercaptoethanol. The cells were maintained at 37 °C in a 50 mL/L CO2 humidified atmosphere. Half of the medium was replaced on d 3. After 5-6 d in culture, cells were collected and enriched by centrifugation over a 135 g/L metrizamide gradient. The purity of DCs fraction was higher than 80% as determined by FACS analysis of surface molecules expression (B7-1, B7-2, MHC-I, MHC-II).
DCs were infected with Ad-c or Ad-p53 (10000 viral particles per cell) for 90 min in 0.5 mL serum-free RPMI 1640 medium supplemented with 20 ng/mL GM-CSF and 10 ng/mL IL-4 in 24-well plates followed by culturing in CCM with the same cytokines for 48 h. This dose was selected after some preliminary experiments, and did not affect cell viability (95% viable after trypan blue staining).
DCs were washed twice in PBS and incubated with 14 μg/mL hamster anti-mouse CD40 monoclonal antibody for 25 min on ice, then washed in RPMI 1640 twice and cultured in 1 mL CCM supplemented with 3.5 μg/mL anti-hamster IgM, 20 ng/mL GM-CSF and 10 ng/mL IL-4 overnight. Four kinds of DCs such as DC, wtp53DC, LyDC, and Lywtp53 were collected and loaded with tumor cell lysates.
DCs were estimated by intracellular staining followed by flow cytometry. Cells were washed in PBS twice, fixed by 2.5 g/L paraformaldehyde solution for 30 min on ice, washed in PBS, permeabilized by 2 g/L Tween 20 for 15 min at 37 °C, washed in PBS, incubated with FITC-labeled mouse anti-mouse p53 antibody or isotype mouse IgG2a for 25 min on ice, washed twice in PBS and analyzed by FACScalibur low cytometer.
To study the expression of surface molecules, DCs were washed twice in PBS, incubated with 1 μg/106 cells FITC-labeled anti-mouse CD11c antibody and PE-labeled anti-mouse I-Ad or anti-mouse B7-1, B7-2, MHC-I, MHC-II antibody for 25 min on ice, washed twice in PBS and analyzed by flow cytometry. Non-specific binding was estimated using FITC-labeled isotype mouse IgG2a, k and PE-labeled isotype mouse IgG2b, k.
DCs were generated from bone marrow of BALB/c mice and infected with adenovirus as described above. Forty-eight hours later cells were washed 3 times in PBS and injected to BALB/c mice (2 × 105 /mouse) 3 to 4 times with a 10-14 d interval. Seven days after the last immunization Mk28 tumor cells (3.5 × 105 /mouse) were inoculated s.c. and the size of the tumor was observed.
The same tumor model was used to evaluate the effectiveness of treatment. Five hundred thousand Mk28 cells were inoculated s.c. shaved backs of BALB/c mice. The treatment was started when tumors reached 4-6 mm in diameter (d 7). Mice were treated with DCs prepared as described above. The treatment was repeated 4 times with 5- to 6-d intervals. Tumor sizes were measured every 3-5 d for 4 wk.
Splenocytes (effector cells) freshly isolated or restimulated for 7 d with 4 kinds of DCs were mixed with different 51Cr-labeled target cells: Mk28 cells pre-incubated for 48 h with 5 ng/mL murine IFN-γ or p815 cells infected with Ad-p53 or Ad-c. After 6-h incubation and harvesting of supernatants, the radioactivity was measured by gamma-counter. To estimate the maximum 51Cr-release, 10 g/L triton X-100 was used. The percentage of cell lysis was calculated as follows: (experimental release-spontaneous release)/(maximum release-spontaneous release) × 100%.
Surface molecules of lywt-p53DC highly expressed were detected by FACS: B7-1 (86.70% ± 0.07%), B7-2 (18.77% ± 0.08%), MCH-I (87.20% ± 0.05%), MCH-II (56.70% ± 0.07%).
To induce an immune response against wild-type p53, we started with three immunizations of BALB/c mice with DCs generated from bone marrow progenitors of syngeneic mice and transduced with Ad-p53 loaded with lysates as described in MATERIALS AND METHODS. We found statistically significant differences in tumor formation between mice immunized with Lywtp53 DCs and control groups (9 mice per group), and no differences between wtp53DC and LyDC(Table 1).
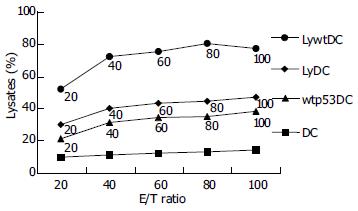
The presence of Mk28-specific CTLs was evaluated in mice immunized with activated Ad-p53-transduced DCs. Six-hour standard CTL assay was performed 1 mo and 1 wk after the last immunization (1 mo after inoculation of Mk28 cells into immunized mice). Freshly isolated splenocytes were mixed with 51Cr-labeled Mk28 cells at different ratios. Splenocytes from mice injected with Ad-c-infected DCs demonstrated no ability to lyse target cells, whereas splenocytes from mice immunized with Ad-p53-transduced DCs demonstrated low, but clearly significant cytotoxicity against Mk28 cells (Figure 1).
The immune protection of Lywtp53DC group was obvious, the tumor size of Lywtp53DC group was smaller than control, DC, wtp53DC and LyDC group on d 13, 16 and 19 (Table 2).
| Group | Tumor diameter after inoculation (mm) | |||
| d 10 | d 13 | d 16 | d 19 | |
| Control | 3.72 ± 0.01 | 6.70 ± 0.02 | 7.10 ± 0.09 | 9.90 ± 0.02 |
| DC | 3.70 ± 0.01 | 5.70 ± 0.08 | 6.10 ± 0.04 | 9.70 ± 0.08 |
| Wtp53DC | 3.07 ± 0.01 | 6.90 ± 0.04 | 7.13 ± 0.03 | 8.22 ± 0.08 |
| LyDC | 4.03 ± 0.04 | 6.13 ± 0.05 | 7.10 ± 0.03 | 8.77 ± 0.05 |
| Lywtp53 | 3.13 ± 0.02 | 3.10 ± 0.31a | 2.73 ± 0.23a | 3.70 ± 0.07a |
Treatment of tumor-bearing mice with Ad-p53-transduced DCs and CTL response are shown in Table 3. On d 15 and 18, the growth rate of tumor in Lywtp53DC group was slower than any of the other group (Table 3).
| Group | Tumor diameter after inoculation (mm) | |||
| d 7 | d 12 | d 15 | d 18 | |
| Control | 5.72 ± 0.02 | 7.88 ± 0.09 | 9.20 ± 0.11 | 11.90 ± 0.09 |
| DC | 4.70 ± 0.03 | 7.71 ± 0.08 | 9.02 ± 0.04 | 10.70 ± 0.68 |
| Wtp53DC | 5.03 ± 0.09 | 6.90 ± 0.09 | 7.13 ± 0.11 | 8.29 ± 0.05 |
| LyDC | 5.06 ± 0.01 | 6.13 ± 0.08 | 7.10 ± 0.05 | 8.77 ± 0.03 |
| Lywtp53 | 5.19 ± 0.09 | 5.10 ± 0.39 | 6.73 ± 0.66a | 6.79 ± 0.77a |
p53 protein is an attractive target for immunotherapy of cancer. Normal cells have very low levels of p53, whereas accumulation of this protein because of mutations or functional inactivation is observed in 50% of human malignancies. This provides, in theory, potential targets for CTLs that recognize class I MHC-bound epitopes[1-3].
In this study we have demonstrated that activated DCs transduced with full-length wild-type p53 loaded with lysates are able to break tolerance to this self-protein and induce potent antitumor response with no detectable autoimmune abnormalities. Wild-type, p53-derived, self-MHC-self-peptide complexes expressed by bone marrow-derived cells in the thymus cause negative selection of immature thymic T cells with a high avidity for such complexes[4,5]. These results in deletion of T cells with sufficient avidity to recognize natural wild-type p53 epitopes presented by MHC class I molecules on tumor cells and thus prevent immune response. Only low avidity CTLs survive during the induction of self-tolerance[6-8].
We suggest here another method of immunotherapy based on the use of full-length wild-type p53. This approach may be devoid of many limitations of peptide-based immunization and would provide a valuable option for clinical trials. Overexpression of wild-type p53 in antigen-presenting cells would allow presentation of several different epitopes. The feasibility of such an approach was shown previously in model experiments in which each of the different minimal epitopes combined to a single fusion protein can be presented separately on the cell surface and be recognized by specific CTLs[9,10].
The study demonstrated that the wild type p53 was transduced to DCs with adenovirus, and the DCs were stimulated by gastric cancer lysates. The T lymphocytes had specific CTL lysis ability induced by Lywt-p53DC loaded with lysates, the CTL lysis rate was as high as 81%. The surface molecules of Lywt- p53DC showed a high expression of B7-1 (86.70% ± 0.07%), B7-2 (18.77% ± 0.08%), MHC-I (87.20% ± 0.05%), MHC-II (56.70% ± 0.07%); There were significant differences in tumor sizes between Lywtp53DC group and any other group. In Lywtp53DC group, the growth rate of tumor was slower than any one of the other group (P < 0.05). These studies showed that Lywtp53 DC had immune protection effect on mice, especially loaded with lysates[11-14].
Because of a polyclonal nature of T cells generated after two rounds of stimulations with p53 DCs, it is possible that some level of cytotoxicity against tumor cells could be mediated by alloreactivity. When the DCs were loaded with lysates of tumor, the lysates will be presented to APC, and the process can increase immunogenicity in the body. DCs were recognized; the specificity of CTLs was enhanced[15,16].
In conclusion, these data indicate that DCs transduced with full-length wild-type p53 loaded with lysates are able to generate a CTL response specifically for tumors with p53 overexpression. These findings demonstrate that this approach may overcome tolerance to self-protein and may serve as a valuable option in cancer immunotherapy.
Edited by Zhu LH Proofread by Chen WW and Xu FM
| 1. | Nikitina EY, Clark JI, Van Beynen J, Chada S, Virmani AK, Carbone DP, Gabrilovich DI. Dendritic cells transduced with full-length wild-type p53 generate antitumor cytotoxic T lymphocytes from peripheral blood of cancer patients. Clin Cancer Res. 2001;7:127-135. [PubMed] |
| 2. | Nagayama H, Sato K, Morishita M, Uchimaru K, Oyaizu N, Inazawa T, Yamasaki T, Enomoto M, Nakaoka T, Nakamura T. Results of a phase I clinical study using autologous tumour lysate-pulsed monocyte-derived mature dendritic cell vaccinations for stage IV malignant melanoma patients combined with low dose interleukin-2. Melanoma Res. 2003;13:521-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Adams M, Navabi H, Jasani B, Man S, Fiander A, Evans AS, Donninger C, Mason M. Dendritic cell (DC) based therapy for cervical cancer: use of DC pulsed with tumour lysate and matured with a novel synthetic clinically non-toxic double stranded RNA analogue poly [I]: poly [C (12) U] (Ampligen R). Vaccine. 2003;21:787-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Shimizu K, Thomas EK, Giedlin M, Mulé JJ. Enhancement of tumor lysate- and peptide-pulsed dendritic cell-based vaccines by the addition of foreign helper protein. Cancer Res. 2001;61:2618-2624. [PubMed] |
| 5. | Eura M, Chikamatsu K, Katsura F, Obata A, Sobao Y, Takiguchi M, Song Y, Appella E, Whiteside TL, DeLeo AB. A wild-type sequence p53 peptide presented by HLA-A24 induces cytotoxic T lymphocytes that recognize squamous cell carcinomas of the head and neck. Clin Cancer Res. 2000;6:979-986. [PubMed] |
| 6. | Huang HL, Wu BY, You WD, Shen MS, Wang WJ. [Influence of dendritic cell infiltration on prognosis and biologic characteristics of progressing gastric cancer]. Zhonghua Zhongliu Zazhi. 2003;25:468-471. [PubMed] |
| 7. | Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Xiangming C, Iwashige H, Aridome K, Hokita S, Aikou T. Clinical impact of intratumoral natural killer cell and dendritic cell infiltration in gastric cancer. Cancer Lett. 2000;159:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Weisbart RH, Miller CW, Chan G, Wakelin R, Ferreri K, Koeffler HP. Nuclear delivery of p53 C-terminal peptides into cancer cells using scFv fragments of a monoclonal antibody that penetrates living cells. Cancer Lett. 2003;195:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Asai T, Storkus WJ, Mueller-Berghaus J, Knapp W, DeLeo AB, Chikamatsu K, Whiteside TL. In vitro generated cytolytic T lymphocytes reactive against head and neck cancer recognize multiple epitopes presented by HLA-A2, including peptides derived from the p53 and MDM-2 proteins. Cancer Immun. 2002;2:3. [PubMed] |
| 10. | Portefaix JM, Rio MD, Granier C, Roquet F, Pau B, Navarro-Teulon I. Peptides derived from the two regulatory domains of p53 are recognized by two p53-activating antibodies. Peptides. 2003;24:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Takenobu T, Tomizawa K, Matsushita M, Li ST, Moriwaki A, Lu YF, Matsui H. Development of p53 protein transduction therapy using membrane-permeable peptides and the application to oral cancer cells. Mol Cancer Ther. 2002;1:1043-1049. [PubMed] |
| 12. | Hoffmann TK, Loftus DJ, Nakano K, Maeurer MJ, Chikamatsu K, Appella E, Whiteside TL, DeLeo AB. The ability of variant peptides to reverse the nonresponsiveness of T lymphocytes to the wild-type sequence p53 (264-272) epitope. J Immunol. 2002;168:1338-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Portefaix JM, Fanutti C, Granier C, Crapez E, Perham R, Grenier J, Pau B, Del Rio M. Detection of anti-p53 antibodies by ELISA using p53 synthetic or phage-displayed peptides. J Immunol Methods. 2002;259:65-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Kanovsky M, Raffo A, Drew L, Rosal R, Do T, Friedman FK, Rubinstein P, Visser J, Robinson R, Brandt-Rauf PW. Peptides from the amino terminal mdm-2-binding domain of p53, designed from conformational analysis, are selectively cytotoxic to transformed cells. Proc Natl Acad Sci USA. 2001;98:12438-12443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Ferriès E, Connan F, Pagès F, Gaston J, Hagnéré AM, Vieillefond A, Thiounn N, Guillet J, Choppin J. Identification of p53 peptides recognized by CD8 (+) T lymphocytes from patients with bladder cancer. Hum Immunol. 2001;62:791-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Petersen TR, Buus S, Brunak S, Nissen MH, Sherman LA, Claesson MH. Identification and design of p53-derived HLA-A2-binding peptides with increased CTL immunogenicity. Scand J Immunol. 2001;53:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |