INTRODUCTION
Leptin, a circulating hormone secreted by adipocytes, is one of the major adipose cytokines acting as an important signaling molecule in energy regulation and food intake, and controls body weight homeostasis[1-3]. It modulates various physiological and pathophysiological states, including lipid metabolism, hematopoiesis, thermogenesis, obesity, and polycystic ovary syndrome[1,4]. Besides, leptin is also involved in obesity and carcinogenesis of some human cancers.
Recent studies suggested that leptin might play an important role in the process of initiation and progression of human cancers[2,3,5-12], but there were a difference and discordance among the results. Circulating levels of leptin were higher in cases of breast cancer[5], colorectal cancer[8], prostate cancer[6], eudiometrical cancer[13], and lower in cases of uterine leiomyomas than the controls[10], or had no difference in breast cancer[14]. One study showed that leptin might play a role in the development of prostate cancer[11]. Another study suggested plasma leptin was not associated with prostate cancer risk[10]. On the other hand, studies suggested that leptin expression was associated with invasive potential of functioning pituitary adenomas[15], and influenced cellular differentiation and the progression of prostate cancer[11]. In vitro experimental studies, leptin not only stimulate proliferation of some human cancer cell lines, including breast cancer cell lines (ZR75-1, MCF-7, T-47D)[2,7,9,16-18], esophageal cancer cell lines (KYSE410, 150), prostate cancer cell lines (DU145 and PC-3)[2,3], acute myelogenous leukemia (AML)[19] cell lines, but also inhibited the growth of some human cancer cell lines, such as pancreas cancer cell lines (PANC-1, Mia-PaCa)[2]. The study results indicated that leptin had divergent effects on human cancer cell proliferation.
Hepatocellular carcinoma (HCC) is one of the most common malignancies in the world, and causes an estimated one million deaths annually. It has a poor prognosis due to its rapid infiltrating growth and complicating liver cirrhosis[20,21]. Epidemiological studies suggested that obesity was a risk factor of HCC complicated with alcoholic liver disease and cryptogenic cirrhosis[22,23]. Clinical investigations showed that serum leptin levels in alcoholic and post-hepatitis liver cirrhosis patients with or without HCC increased significantly compared with those in control subjects[24-30], suggesting that leptin is involved in the etiogenesis of hepatocellular carcinoma (HCC) in cirrhotic patients. Therefore, we hypotheses that leptin might be associated with the growth of liver cells and HCC cells, and played a role in carcinogenesis of hepatocellular carcinoma, but such a study has been reported. In order to confirm this hypothesis, the expression of leptin in human HCC and adjacent non-tumorous liver tissues was analyzed by immunohistochemical staining, and the effect of leptin on proliferation of normal liver cells and HCC cells in vitro was studied in this study.
MATERIALS AND METHODS
Immunohistochemical analysis of leptin and Ob-R expressions in human HCC and adjacent non-tumorous liver tissues
Patients and samples HCC specimens and corresponding adjacent non-tumorous liver tissues were collected from 36 patients surgically treated in West China Hospital of Sichuan University in China in 2000, all the patients were informed and consented to use their samples for this study. Of the 36 patients, 29 (80.56%) were males, 7 (19.44%) were females, their age ranged from 27 to 68 years (mean, 49.4 years), Twenty patients (20/36, 55.56%) were infected with HBV, 32 (32/36, 88.89%) were complicated with liver dysfunction, and 10 (10/36, 27.78%) with liver cirrhosis. None had any treatment before the surgical operation. All patients were diagnosed as HCC. The tissue specimens were routinely processed, formalin-fixed, and paraffin- embedded. Paraffinized sections were made for hematoxylin and eosin (HE) and immunohistochemical staining.
Reagents Ob (A-20) and Ob-R (H-300) rabbit anti-human polyclonal antibodies were purchased from Santa Cruz Biotechnology, USA. S-P immunohistochemical staining kit (SP9001) was purchased from Beijing Zhongshan Biological Technology Ltd.
Method Leptin protein and Ob-R were detected immunohito- chemically with S-P method. Briefly, tissue sections were deparaffinized and rehydrated through graded alcohols. Antigen retrieval was performed by heating in microwave oven in 0.1 mol/L citrate buffer (pH6). Then, endogenous peroxidase activity was blocked with 30 mL/L H2O2, and after treatment with normal goat serum, the sections were incubated with Ob (A-20) or Ob-R (H-300) rabbit anti-human polyclonal antibodies at a dilution of 1:100, overnight at 4°C, with biotinylated second antibody for 20 min, and with streptavidin/peroxidase for 30 min at room temperature. Subsequently, the sections were subjected to color reaction with 0.2 g/L 3,3 diaminobenzidine tetrahydrochloride containing 0.05 mL/L H2O2 in PBS (pH7.4), and counterstained with hematoxylin lightly. In each staining run, a known leptin positive sample of fat tissue was added as a positive control, and a section of the same fat tissue incubated in PBS instead of Ob or Ob-R was included as a negative control.
Semi-quantitative evaluation was used to determine positively expressed cells by viewing 10 vision fields at × 400. Negative (-) indicated cells were stained less than 10%, mildly positive (+) showed 11%-25% cells were stained, moderately positive (++) demonstrated 26%-50% cells were stained, strongly positive (+++) revealed over 50% cells were stained. The intensity of staining in positive cells was compared with the negative control and scored as follows: negative (-), weak (+), moderate (++), strong (+++), the last three grades were all regarded as positive.
Proliferation assay in vitro
Reagents Chang liver cell line (ATCC CCL13) and liver cancer cell line SMMC-7721 were obtained from Shanghai Cell Biology Institute of Chinese Academy of Sciences. Human recombinant leptin was a product from Signa-Aldrich, Inc. USA. RPMI-1640 was the product of Gibco/BRL (Invitrogen Corporation, USA). Fetal bovine serum (FBS) was purchased from Huaxi Biology Institute (Chengdu, China). Trypsin, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), and DMSO were purchased from the Sino-American Biotechnology Company of Beijing.
Cell culture Both Chang liver cell line and SMMC-7721 cell line were cultured in RPMI-1640 medium supplemented with 100 mL/L FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin, at 37°C in a humidified atmosphere containing 50 mL/L CO2.
Proliferation assay (MTT) Chang liver cells and SMMC-7721 cells were suspended at a concentration of 5 × 104/mL Then 200 μL of the cell suspension was placed in each well of a replicate 96-well microtiter plate. The cells were allowed to adhere overnight. Then different concentrations (10, 20, 40, 80, 160, 320 ng/mL) of leptin were added to each kind of cells. MTT assay was performed after 1-, 3-, 5-d growth. A 20 μL of 5 mg/mL of MTT was added to each well followed by incubation for 4 h at 37°C. Formazan products were solubilized with DMSO; A values were read with an enzyme-linked immunosorbent assay reader (Bio-Tek, Houston, USA) at wavelength of 570 nm. All experiments were performed in triplicate. The effect of leptin on proliferation of Chang liver cells and SMMC-7721 cells were expressed as cell survival rates: A value of the treated group ÷A value of control group × 100 %.
Statistics analysis
The data of immunohitochemical staining were analyzed with chi-square test. The data of cell proliferation assay were analyzed with variance analysis. P < 0.05 was considered statistically significant.
RESULTS
Leptin expression in human HCC and adjacent non-tumorous liver tissues
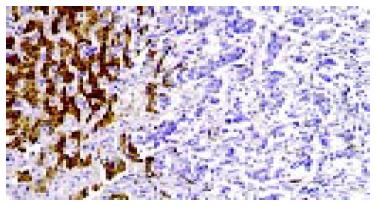
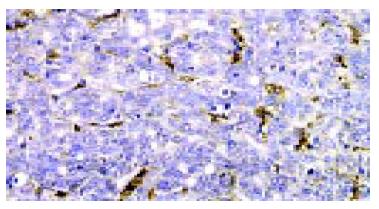
The expressed Ob gene product, leptin was detected in all 36 specimen from adjacent non-tumorous liver tissues (36/36,100%), with moderate (++) to strong (+++) intensity. The expression rate in HCC was 72.22% (26/36), but the intensity was weaker (+) than that in adjacent non-tumorous liver tissues (Figure 1), a significant difference in leptin expression was found between adjacent non-tumorous liver tissues and HCC (P < 0.05). Ten of 36 specimens from HCC (10/36, 27.78%) even lost the expression of leptin, but Kupffer cells and vascular endothelial cells expressed high levels of leptin (Figure 2).
Figure 1 Expression of leptin in adjacent non-tumorous liver live cells (left) and absent expression in HCC cells (right).
S-P immunohistochemical staining × 200.
Figure 2 Absent expression of leptin in HCC cells, Kupffer cells and vascular endothelial cells expressed high levels of leptin.
S-P immunohistochemical staining × 400.
Ob-R expression in human HCC and adjacent non-tumorous liver tissues
Thirty out of 36 specimens from adjacent non-tumorous liver tissues (30/36, 83.33%) were positive for Ob gene receptor product, with a moderate (++) to strong (+++) intensity. Only 11 of 36 specimens from HCC (11/36, 30.56%) were positive, with a weak (+) intensity. The expression of Ob-R was significantly lower than that of adjacent non-tumorous liver tissues (P < 0.05).
Effect of leptin on the proliferation of Chang liver cells in vitro
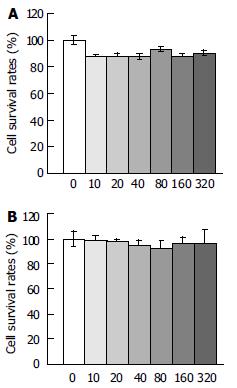
Leptin inhibited the proliferation of Chang liver cells on the 5th d after leptin treatment at various concentrations. The cell survival rate was 10%-13% lower than that of the untreated cells (Figure 3A), although without statistical significance (P > 0.05).
Figure 3 Effect of leptin at different concentrations (ng/mL) on proliferation of Chang liver cells and liver cancer cells on d 5 (MTT assay).
A: Effect of leptin at different concentrations (ng/mL) on proliferation of Chang liver cells on d 5 (MTT assay), B: Effect of leptin at different concentrations (ng/mL) on proliferation of liver cancer cells on d 5 (MTT assay).
Effect of leptin on proliferation of SMMC-7721 cells in vitro
Leptin had little effect on the proliferation of liver cancer cells (Figure 3B), the cell survival rate on each day after leptin treatment was lightly lower than that of the controls, but no statistical significance was found (P > 0.05).
DISCUSSION
It has found that certain cancers are associated with obesity, including breast, prostate, colon, uterus, renal cell, pancreas carcinoma and esophagus adenocarcinoma[2,22,31]. Leptin, an Ob gene product, could control body weight homeostasis through food intake and energy expenditure, and was closely associated with obesity[1-3]. Although epidemiological observation showed that obesity was a risk factor of HCC complicated with alcoholic liver disease and cryptogenic cirrhosis[22,23,32], clinical investigations have identified that serum leptin levels in alcoholic and post-hepatitis liver cirrhosis patients with or without HCC are increased significantly in comparison with those in control subjects[22,24-30]. There was no study on the role of leptin in carcinogenesis of human hepatic cellular carcinoma[2,22-30].
The present study investigated the expression of leptin and its receptor Ob-R in both adjacent non-tumorous liver tissues and HCC tissues with immunohistochemical staining, and found that adjacent non-tumorous liver cells expressed higher levels of leptin and its receptor than corresponding HCC cells, about one fourth of HCC tissues lost the expression of leptin, and two thirds had no Ob-R expression (Figures 1, Figures 2). These findings suggest that leptin could act in vivo as a paracrine/endocrine growth factor towards hepatocytes, and played a possible role in the process of initiation and progression of HCC, which could thus contribute to the explanation of the reasons why alcoholic and post-hepatitis liver cirrhosis patients with or without HCC had high levels of serum leptin[24-30] and the reasons why obesity was a risk factor of developing HCC[22,23].
In proliferation assay in vitro, leptin had differential effects on the proliferation of human cancer cells. Tt caused growth potentiation in breast, esophagus, and prostate cancer cells, but inhibited the proliferation of pancreatic cancer cells[2,19,32]. In MCF-7 cells, leptin and high glucose stimulated cell proliferation as demonstrated by the increases in DNA synthesis and expression of cdk2 and cyclin D1. However, they had no significant effect on the proliferation of multidrug-resistant human breast cancer NCI/ADR-RES cells[16]. In this study, leptin alone showed some inhibitory effect on the proliferation of normal Chang liver cells, and no effect on liver cancer SMMC-7721 cells. However, Chang liver cell line was originally thought to be derived from normal liver tissue, but was subsequently found on the basis of isoenzyme analysis, HeLa marker chromosomes, and DNA fingerprinting, to have HeLa cell contamination when it was established (http://www.atcc.org). Therefore, these findings in vitro might be associated with different biological characteristics of cancer cells to react to leptin treatment, and/or the organ derivation of cancer cell lines[2]. In addition, Chang liver cell line was not a good control of normal liver cells.
In vivo animal experiments findings showed that Leptin was a growth factor that could stimulate proliferation of colonic epithelial cells in mice[33]. It is also a profibrogenic cytokine and could play a key role in liver fibrosis[34]. ob/ob mice having a naturally occurring mutation in the ob gene that prevents the synthesis of leptin had an increased incidence of HCC[31]. This study showed that leptin had some inhibitory effect on the proliferation of normal Chang liver cells and no effect on the proliferating of on liver cancer SMMC-7721 cells (Figures 3A,3B), and adjacent non-tumorous liver tissues could express higher levels of leptin and its receptor than the corresponding HCC cells (Figure 1). It could be assumed that leptin acting as an inhibitor and/or promoter, was involved in the process of carcinogenesis and progression of human HCC. In addition, the high levels of leptin expression in Kupffer cells and vascular endothelial cells might be associated with liver cirrhosis[24,27,35,36], and angiogenesis of cancer tissues[1,14,37], because these lesions might deteriorate HCC.
In summary, a preliminary investigation of the possible involvement of leptin in carcinogenesis of human hepatocellular carcinoma was performed in this study to elucidate the etiology and carcinogenesis of HCC. To the best of our knowledge[2,20-30], this is the first report on involvement of leptin in HCC. The presence of leptin and its receptor in adjacent non-tumorous liver and HCC cells, and the effects of leptin on proliferation of normal Chang liver cells and liver cancer cells may provide new insights into the carcinogenesis and progression of human HCC.