Published online Aug 15, 2004. doi: 10.3748/wjg.v10.i16.2447
Revised: December 6, 2003
Accepted: December 16, 2003
Published online: August 15, 2004
AIM: Gastrointestinal autonomic nerve tumors are uncommon stromal tumors of the intestinal tract. Their histological appearance is similar to that of other gastrointestinal stromal tumors. We report two cases and performed an analysis of the literature by comparing our findings with the available case reports in the medical literature.
METHODS: Two patients were admitted with abdominal tumor masses. One occurred in the stomach with large multiple liver metastases and the second originated in Meckel´s diverticulum. The latter site has never been reported previously. Both patients underwent surgery. In one patient gastrectomy, right liver resection and colon transversum resection were performed to achieve aggressive tumor debulking. In the other patient the tumor bearing diverticulum was removed.
RESULTS: Postoperative recovery of both patients was uneventful. Histological examination, immunohistochemical analysis and electron microscopy revealed the diagnosis of a gastrointestinal autonomic nerve tumor. The patient with the tumor in Meckel’s diverticulum died 6 mo after surgery because of pneumonia. The patient with liver metastases have been alive 13 years after initial tumor diagnosis and 7 years after surgery with no evidence of tumor progression. In light of our results, we performed a thorough comparison with available literature reports.
CONCLUSION: Radical surgical resection of gastrointestinal autonomic nerve tumors seems to be the only available curative approach to date, and long term survival is possible even in large metastasized tumors.
- Citation: Stift A, Friedl J, Gnant M, Herbst F, Jakesz R, Wenzl E. Gastrointestinal autonomic nerve tumors: A surgical point of view. World J Gastroenterol 2004; 10(16): 2447-2451
- URL: https://www.wjgnet.com/1007-9327/full/v10/i16/2447.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i16.2447
Gastrointestinal autonomic nerve tumors (GANTs) were first described by Herrera et al[1] in 1984. The authors used the term plexosarcoma to describe this entity. In the last decade several investigators have recognized the distinct characteristics of this type of tumor[2-6]. It consists of a subgroup of gastrointestinal stromal tumors (GISTs) with a specific ultrastructural appearance, suggesting that it originates from neurons of the enteric plexus. Histologically, GANTs are usually low-grade spindle-cell neoplasms but cannot be definitely distinguished from stromal tumors or other neurogenic tumors by histological or immunohistochemical features. To date, the diagnosis of GIST is strongly suggested by immunostaining for the transmembrane tyrosine kinase receptor CD117 and c-kit gene mutations[7,8]. Partly based on these findings GISTs have been defined as tumors of the interstitial cells of Cajal[9]. Because GANTs also often showed CD117 staining some authors considered GISTs to be identical to GANTs[10]. Eyden et al[6] have recently studied 82 gastrointestinal stromal tumors in terms of their cellular differentiation by using electron microscopy. They demonstrated that electron microscopy was needed to establish the diagnosis of GANTs and to exclude other gastrointestinal stromal tumors. We performed an analysis of the literature to compare our patients with the published data. The literature search yielded more than 120 published cases of GANTs, including the two cases presented in this report[1-6,11-38].
This female patient was 22-year-old when she first noted fatigue, loss of appetite and epigastric discomfort in 1990. On physical examination she had a palpable epigastric mass. Upper gastrointestinal series and computed tomography (CT) suggested that the mass was a large gastric tumor. Endoscopy revealed several large masses protruding into the stomach without erosion of the overlying mucosa. Laparotomy showed that the entire stomach was infiltrated with nodular masses. In addition, the liver demonstrated multiple nodules. Intraoperatively obtained frozen sections revealed a leiomyosarcoma. Due to the poor prognosis of the disease and the extensive tumor burden the surgeon decided not to remove any tumor mass. The patient was discharged without any further treatment. Histological features and immunohistochemical characteristics were determined and found to be analogous to the results obtained six years later. Ultrastructural examination was not performed. At the time this case was diagnosed, only 10 GANTs had been documented in the literature.
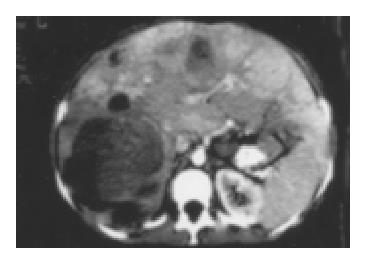
Six years later the patient was still alive and was admitted to our hospital after an extensive weight loss (she weighed 38 kg at the time of admission). The patient complained of progressive abdominal discomfort and dyspnea. On physical examination the abdominal wall was extremely distended by a large palpable epigastric mass. Diagnostic studies including CT scan confirmed the known abdominal tumor. On the CT image the liver foci were partly solid and partly liquid (Figure 1).
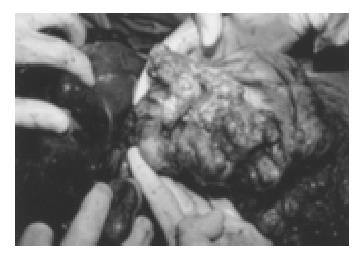
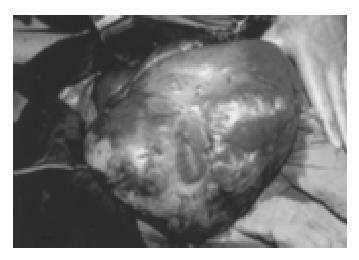
Again, a laparotomy was performed. The intraoperative findings were similar to those obtained 6 years earlier. By then, the gastric tumor invaded the transverse mesocolon. Maximum debulking was achieved by total gastrectomy, transverse colon resection and right hepatectomy, leaving only small tumor deposits in the left liver. The resected gastric tumor mass measured approximately 40 cm × 25 cm × 20 cm in aggregate and the liver foci covered 25 cm × 12 cm × 12 cm (Figure 2, Figure 3). After an uneventful recovery the patient was discharged after 3 wk and returned to work 5 wk later. CT series obtained 3, 6 and 12 mo after the operation showed no measurable expansion of the remaining foci in the left liver. A CT scan performed 24 mo after the operation revealed spontaneous partial regression of the metastatic liver foci. Seven years after surgery, the patient was still in excellent clinical condition and free of discomfort. In addition, the patient delivered a healthy boy in 1997.
Microscopically, the resected gastric tumor chiefly consisted of plump spindle cells with distinct nuclear pleomorphism. In some areas 15 mitotic figures per 10 high power fields were identified. Immunohistochemical studies showed positive staining with antibodies to neuron specific enolase (NSE) and vimentin. There was also a weak focal staining for S 100 and HISL-19. No detectable staining for chromogranin A, 1 A 4, HHF 35, EMA, MNF 116, CAM 5.2, JC 70a and CD117 was observed. Just a small part of the tumor mass showed positive staining with an antibody to CD 34. Microscopically, the resected metastatic liver tumor also mainly consisted of plump spindle cells. In contrast to the original tumor mass, immunohistochemical studies showed 100 percent positive staining for CD 34 but negative staining for CD 117. CD 117 staining was performed a few weeks ago in order to take into account newest drug developments like tyrosine kinase inhibitors as a potential treatment option. Approximately 80% of the sampled tumors were necrotic. Ultrastructural examination showed neuron-like cells with long cytoplasmatic processes and dense core neurosecretory granules. Thus, the diagnosis of a gastrointestinal autonomic nerve tumor (GANT) was made.
A 68-year-old man was admitted to the hospital with a one month history of fever and weight loss of 4 kg. No abnormal findings were registered on physical examination. Computerized tomography showed a decaying liver tumor. The Mendel-Mantoux reaction was highly positive. Therefore the liver focus was considered to be tuberculotic. Tuberculostatic triple-drug therapy was administered for 6 mo. After 12 mo, a CT scan of the abdomen and upper gastrointestinal series showed a normal liver with no pathological signs. An additional new finding was suggestive of a tumor of the ileum. Laparotomy revealed neoplastic nodular masses (9 cm×6 cm) originating from Meckel’s diverticulum. The tumor was resected along with an ileal segment. The patient had an uneventful recovery and was discharged 10 d postoperatively. Light microscopy revealed that the tumor was composed of interlacing fascicles of plump, pleomorphic spindle cells, partly showing a storiform and epitheloid pattern. Less than one mitotic figure per 10 high-power fields was identified. Immunohistochemical stainings for vimentin, S-100 and neuron specific enolase (NSE) were positive, whereas staining for Lu 5, CAM 5.2, MNF 116, EMA, desmin, chromogranin, 1A4, Q-BENT10, JC70A, HMB-45, PGM1, HHF35 and CD117 was negative.
Ultrastructural examination showed neuron-like cells with long branching cytoplasmatic processes. Dense-core granules were scarce. No basal lamina, dense bodies, or pinocytotic vesicles were found.
Six months later the patient died of a severe pneumonia. A post-mortem examination showed no intestinal abnormalities or recurrence of disease.
Gastrointestinal autonomic nerve tumors represent a rare distinct subcategory of gastrointestinal stromal tumors. First described by Herrera et al[1-6,11-38] in 1984 as plexosarcoma supposedly originating from the enteric autonomic plexus, more than 120 cases including our own have been documented in the literature. These tumors were found in patients of a wide age range (10 to 85 years) with a median age of 58 years. Seventy-nine per cent of patients were older than 50 years. The tumors were found slightly more often in males (62 males vs 58 females). The reported clinical signs and symptoms are shown in Table 1. In the majority of cases, the primary site of the tumor was the stomach, duodenum, jejunum and ileum, but the esophagus[21,22], rectum[26,34], bladder[30] or colon[31] have also been reported in some cases (Table 2).
| Clinical symptoms | No. of patients | Percent of patients |
| Abdominal pain | 32 | 41 |
| Meaena | 19 | 24 |
| Abdominal mass | 10 | 13 |
| Weight loss | 10 | 13 |
| Vomiting | 9 | 12 |
| Fatigue | 7 | 9 |
| Fever | 5 | 6 |
| Anemia | 4 | 5 |
| Obstructive jaundice | 2 | 2 |
| No clinical symptoms | 5 | 6 |
| Location | No. of patients | Percent of patients |
| Stomach | 45 | 37.5 |
| Jejunum | 26 | 22.0 |
| Ileum | 21 | 17.5 |
| Duodenum | 12 | 10.0 |
| Retroperitoneum | 4 | 3.3 |
| Mesentery | 3 | 2.5 |
| Esophagus | 2 | 1.6 |
| Peritoneum | 2 | 1.6 |
| Rectum | 2 | 1.6 |
| Colon | 1 | 0.8 |
| Bladder | 1 | 0.8 |
| Omentum | 1 | 0.8 |
On imaging studies, the tumor often presents as a large and lobulated solid mass, with areas of necrosis. It is locally invasive. Radiological techniques did not permit a distinction between GANTs and other gastrointestinal stromal tumors[28,33].
The greatest dimension of the tumor was determined in 81 cases, and ranged from 1.5 cm to 40 cm (median 10.4 cm). Eighty per cent was larger than 5 cm and 37% had one dimension larger than 10 cm. The bulky tumor masses were predominantly cystic and necrotic. In two cases an abscess was present in the tumor[17,29]. Some of these GANTs were observed in patients with Carney’s triad (3 cases)[16,17], neurofibromatosis (3 cases)[15,20,25], and adrenal ganglioneuroma (1 case)[20].
The tumors were usually intramuscular-intramural, well demarcated, but not encapsulated, unilocated or multilocated masses of variable consistency, with or without haemorrhage and necrosis.
Histologically, the reported tumors showed a variety of patterns, none of which was specifically diagnostic. Most lesions were spindle cell tumors with features resembling either smooth muscle tumors or neurogenic tumors. Epitheloid cells were also detected, usually as a minor population. The cellular arrangement was either whorled, patternless, fascicular, palisaded or storiform. Generally, the tumor cells had an eosinophilic cytoplasm. Pleomorphism was not significant and mitosis ranged from 1 to 23/10 high-power fields.
Results of immunohistochemical studies demonstrated that the tumor was most often reactive to vimentin and other markers of nerve tissues such as neuron-specific enolase (NSE), synaptophysin, S-100 protein, neurofilament, and chromogranin A (Table 3). These proteins are normally expressed by neurons from the autonomic enteric nerve plexus, supporting a histogenesis of GANTs from enteric autonomic plexuses of Meissner or Auerbach. The muscle marker desmin and muscle-specific actin could not be demonstrated, although focal alpha-SMA positivity was seen in 7 reported cases[24,27]. Except for one study (2 cases) focal staining for cytokeratins (CAM5.2) was found to be negative[26]. GANTs usually lacked smooth muscle cell features[1,2,11].
| Antigens | No. of cases studied | No. Positive cases | Percent of positivity |
| Vimentin | 103 | 95 | 92 |
| Neuron-specific | 117 | 105 | 90 |
| Enolase | |||
| Chromogranin A | 92 | 10 | 11 |
| Synaptophysin | 105 | 33 | 31 |
| S-100 protein | 117 | 44 | 38 |
| Neurofilaments | 87 | 14 | 16 |
| Vasoactive intestinal peptide | 25 | 5 | 20 |
Over the last two years GANTs were also tested for CD117 and almost all of them were positive[10].
Ultrastructural studies of all the reported cases revealed features suggestive of myenteric plexus in origin. The diagnostic ultrastructural features included the presence of long, closely opposed cell processes containing intermediate filaments, dense-core neurosecretory granules, microtubules, and synapse-like structures with variable numbers of neurosecretory granules and small vesicles. The essential ultrastructural criteria applied for the diagnosis of GANT in all reported cases included neurosecretory granules and intermediate filaments. It should be noted that five reported GANTs showed weak signs of smooth-muscle morphology. Therefore, the presence of smooth muscle cell features might also suggest GANT[6].
The primary therapy in almost all reported cases was surgical resection or debulking, in 2 cases preceded by radiotherapy, and in nine cases preceded by chemotherapy. Three patients received chemotherapy alone. There was no evidence of clinical response using chemotherapy or radiation. As demonstrated in our report, aggressive tumor debulking without any further treatment could also be considered suitable in terms of prolonged survival and quality of life.
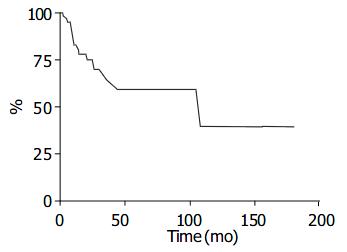
According to published reports, 8 patients out of 67 (12%) had metastases at clinical presentation and 18 patients (27%) developed metastases during follow up (Table 4). The estimated survival curve of the clinically documented cases (n = 67) is shown in Figure 4.
| Metastases at clincal presentation | Metastases in clinical follow up | ||||
| No. of patients | % | No. of patients | % | ||
| Location | 8 | 12 | Location | 18 | 27 |
| Liver | 7 | 10 | Liver | 6 | 9 |
| Retroperitoneal 4 | 6 | ||||
| Lymph node | 2 | 3 | Local recurrence | 5 | 7 |
| Omentum | 3 | 4 | |||
| Omentum | 2 | 3 | Mesentery | 2 | 3 |
| Peritoneum | 2 | 3 | |||
Gastrointestinal autonomic nerve tumors occurred with an estimated frequency of 1% of all malignant gastrointestinal tumors and up to 25% of gastrointestinal stromal tumors[6]. In retrospective studies of collected pathological specimens, several investigators found that light microscopic studies yielded ambiguous results and ultrastructural examination was required in order to establish an accurate diagnosis of gastrointestinal autonomic nerve tumor[6,20,26].
Based on these observations we believe that GANTs are probably more common than previously thought. The rarity of GANT may therefore be a consequence of the unavailability of routine electron microscopic analysis. The exact biological behavior of GANTs is not fully elucidated because of the limited number of reported cases, but it appears that, despite their low grade malignant histological appearance, most GANTs had an uncertain and a poor prognosis in case of metastases. With respect to one of our patients long term survival was possible even with metastatic diseases. Preliminary data indicated no significant correlation between treatment, tumor site and clinical outcomes[2,15].
Some authors suggested that GANTs should be regarded as a type of GISTs because of their mostly identical c-kit mutations[10,39]. Others argued that this common feature might not justify considering GIST and GANT as the same entity because of the neuronal nature of GANT[6]. The recent observation that most GANTs were CD117 positive could have an important clinical impact, since tyrosine kinase inhibitors have yielded good responses in other CD117 positive tumor entities. To date there are no reports on the use of tyrosine kinase inhibitors in CD 117 positive GANTs, but promising data for c-kit positive GISTs which were successfully treated with tyrosine kinase inhibitors have recently been published[39].
Radical surgical resection appears to be the most promising and solely curative treatment regimen for gastrointestinal autonomic nerve tumors. However, as demonstrated in case 1, patients with metastases might also survive for a long time. Some studies in the literature report had similar findings. We therefore suggest that even in cases of large tumor masses, noncurative, aggressive surgical tumor debulking is potentially useful to improve the patient’s quality of life. For metastatic CD117 positive tumors tyrosine kinase inhibitors might be an appropriate palliative treatment approach. As demonstrated in case 2, GAN tumors could also involve unusual anatomic sites like Meckel’s diverticulum.
To gain more information about this distinct type of GISTs, careful ultrastructural and immunohistochemical studies of all GIST cases would be beneficial. We are aware that ultrastructural examination is not available at every clinical pathology unit. However, we propose that pathologists harvest small specimens of the tumor for analysis at specialized institutions. An accurate histological diagnosis seems to be essential in order to ensure that all patients receive adequate and timely treatment and furthermore to learn more about this tumor and its biological behavior especially with respect to newly developed drugs like tyrosine kinase inhibitors. To date there is no difference in the surgical treatment of GANTs and GISTs. But there is some evidence that these tumors are from different origins and may therefore exhibit distinct biological behaviors. However, due to the limited number of reported GANTs further investigations would be necessary to fully characterize these tumors with respect to future treatment decisions.
We thank Christine Brostjan, PhD for critical review of the manuscript.
Edited by Wang XL Proofread by Zhu LH and Xu FM
| 1. | Herrera GA, Pinto de Moraes H, Grizzle WE, Han SG. Malignant small bowel neoplasm of enteric plexus derivation (plexosarcoma). Light and electron microscopic study confirming the origin of the neoplasm. Dig Dis Sci. 1984;29:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 97] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Herrera GA. Small bowel neoplasm. Dig Dis Sci. 1985;30:698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Walker P, Dvorak AM. Gastrointestinal autonomic nerve (GAN) tumor. Ultrastructural evidence for a newly recognized entity. Arch Pathol Lab Med. 1986;110:309-316. [PubMed] |
| 4. | Tortella BJ, Matthews JB, Antonioli DA, Dvorak AM, Silen W. Gastric autonomic nerve (GAN) tumor and extra-adrenal paraganglioma in Carney's triad. A common origin. Ann Surg. 1987;205:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Dvorak AM. Gut autonomic nerve (GAN) tumors. In: Watanabe S, Wolf M, Sommers SC, eds. Digestive disease pathology. Vol 2. Philadelphia WB Saunders. 1989;49-66. |
| 6. | Eyden B, Chorneyko KA, Shanks JH, Menasce LP, Banerjee SS. Contribution of electron microscopy to understanding cellular differentiation in mesenchymal tumors of the gastrointestinal tract: a study of 82 tumors. Ultrastruct Pathol. 2002;26:269-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Miettinen M, Sobin LH, Sarlomo-Rikala M. Immunohistochemical spectrum of GISTs at different sites and their differential diagnosis with a reference to CD117 (KIT). Mod Pathol. 2000;13:1134-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 357] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 8. | Schmid S, Wegmann W. Gastrointestinal pacemaker cell tumor: clinicopathological, immunohistochemical, and ultrastructural study with special reference to c-kit receptor antibody. Virchows Arch. 2000;436:234-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259-1269. [PubMed] |
| 10. | Lee JR, Joshi V, Griffin JW, Lasota J, Miettinen M. Gastrointestinal autonomic nerve tumor: immunohistochemical and molecular identity with gastrointestinal stromal tumor. Am J Surg Pathol. 2001;25:979-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Herrera GA, Cerezo L, Jones JE, Sack J, Grizzle WE, Pollack WJ, Lott RL. Gastrointestinal autonomic nerve tumors. 'Plexosarcomas'. Arch Pathol Lab Med. 1989;113:846-853. [PubMed] |
| 12. | Walsh NM, Bodurtha A. Auerbach's myenteric plexus. A possible site of origin for gastrointestinal stromal tumors in von Recklinghausen's neurofibromatosis. Arch Pathol Lab Med. 1990;114:522-525. [PubMed] |
| 13. | MacLeod CB, Tsokos M. Gastrointestinal autonomic nerve tumor. Ultrastruct Pathol. 1991;15:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Pinedo Moraleda F, Martínez González MA, Ballestín Carcavilla C, Vargas Castrillón J. Gastrointestinal autonomic nerve tumours: a case report with ultrastructural and immunohistochemical studies. Histopathology. 1992;20:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Lauwers GY, Erlandson RA, Casper ES, Brennan MF, Woodruff JM. Gastrointestinal autonomic nerve tumors. A clinicopathological, immunohistochemical, and ultrastructural study of 12 cases. Am J Surg Pathol. 1993;17:887-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 123] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Perez-Atayde AR, Shamberger RC, Kozakewich HW. Neuroectodermal differentiation of the gastrointestinal tumors in the Carney triad. An ultrastructural and immunohistochemical study. Am J Surg Pathol. 1993;17:706-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Thomas JR, Mrak RE, Libuit N. Gastrointestinal autonomic nerve tumor presenting as high-grade sarcoma. Case report and review of the literature. Dig Dis Sci. 1994;39:2051-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Segal A, Carello S, Caterina P, Papadimitriou JM, Spagnolo DV. Gastrointestinal autonomic nerve tumors: a clinicopathological, immunohistochemical and ultrastructural study of 10 cases. Pathology. 1994;26:439-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Kodet R, Snajdauf J, Smelhaus V. Gastrointestinal autonomic nerve tumor: a case report with electron microscopic and immunohistochemical analysis and review of the literature. Pediatr Pathol. 1994;14:1005-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Dhimes P, López-Carreira M, Ortega-Serrano MP, García-Muñoz H, Martínez-González MA, Ballestín C. Gastrointestinal autonomic nerve tumours and their separation from other gastrointestinal stromal tumours: an ultrastructural and immunohistochemical study of seven cases. Virchows Arch. 1995;426:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Lam KY, Law SY, Chu KM, Ma LT. Gastrointestinal autonomic nerve tumor of the esophagus. A clinicopathologic, immunohistochemical, ultrastructural study of a case and review of the literature. Cancer. 1996;78:1651-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Shek TW, Luk IS, Loong F, Ip P, Ma L. Inflammatory cell-rich gastrointestinal autonomic nerve tumor. An expansion of its histologic spectrum. Am J Surg Pathol. 1996;20:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Ojanguren I, Ariza A, Navas-Palacios JJ. Gastrointestinal autonomic nerve tumor: further observations regarding an ultrastructural and immunohistochemical analysis of six cases. Hum Pathol. 1996;27:1311-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Shanks JH, Harris M, Banerjee SS, Eyden BP, Joglekar VM, Nicol A, Hasleton PS, Nicholson AG. Mesotheliomas with deciduoid morphology: a morphologic spectrum and a variant not confined to young females. Am J Surg Pathol. 2000;24:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Sakaguchi N, Sano K, Ito M, Baba T, Fukuzawa M, Hotchi M. A case of von Recklinghausen's disease with bilateral pheochromocytoma-malignant peripheral nerve sheath tumors of the adrenal and gastrointestinal autonomic nerve tumors. Am J Surg Pathol. 1996;20:889-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Erlandson RA, Klimstra DS, Woodruff JM. Subclassification of gastrointestinal stromal tumors based on evaluation by electron microscopy and immunohistochemistry. Ultrastruct Pathol. 1996;20:373-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Matsumoto K, Min W, Yamada N, Asano G. Gastrointestinal autonomic nerve tumors: immunohistochemical and ultrastructural studies in cases of gastrointestinal stromal tumor. Pathol Int. 1997;47:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Jain KA, Gerscovich EO, Goodnight JJ. Malignant autonomic nerve tumor of the duodenum. AJR Am J Roentgenol. 1997;168:1461-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Honda K, Mikami T, Ohkusa T, Takashimizu I, Fujiki K, Araki A, Shimoi K, Enomoto Y, Ariake K, Miyasaka N. Gastrointestinal autonomic nerve tumor with giant abscess. A case report and literature review. J Clin Gastroenterol. 1997;24:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Reid I, Suvarna SK, Wagner BE, Rogers K. Plexosarcoma of the bladder. Eur J Surg Oncol. 1997;23:463-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Donner LR. Gastrointestinal autonomic nerve tumor: a common type of gastrointestinal stromal neoplasm. Ultrastruct Pathol. 1997;21:419-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Minni F, Casadei R, Santini D, Verdirame F, Zanelli M, Vesce G, Marrano D. Gastrointestinal autonomic nerve tumor of the jejunum. Case report and review of the literature. Ital J Gastroenterol Hepatol. 1997;29:558-563. [PubMed] |
| 33. | Rueda O, Escribano J, Vicente JM, Garcia F, Villeta R. Gastrointestinal autonomic nerve tumors (plexosarcomas). is A radiological diagnosis possible? Eur Radiol. 1998;8:458-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Lev D, Kariv Y, Messer GY, Isakov J, Gutman M. Gastrointestinal autonomic nerve (GAN) tumor of the rectum. J Clin Gastroenterol. 2000;30:438-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Kerr JZ, Hicks MJ, Nuchtern JG, Saldivar V, Heim-Hall J, Shah S, Kelly DR, Cain WS, Chintagumpala MM. Gastrointestinal autonomic nerve tumors in the pediatric population: a report of four cases and a review of the literature. Cancer. 1999;85:220-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 36. | Tornóczky T, Kálmán E, Hegedûs G, Horváth OP, Sápi Z, Antal L, Jáksó P, Pajor L. High mitotic index associated with poor prognosis in gastrointestinal autonomic nerve tumour. Histopathology. 1999;35:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Giessling U, Puffer E, Ludwig K. [Gastrointestinal autonomic nerve tumor (GANT)--a rate tumor of the ileum]. Chirurg. 2001;72:600-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 38. | Beck A, Jonas J, Frenzel H, Bähr R. [Gastrointestinal autonomic nerve tumor]. Zentralbl Chir. 2001;126:702-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 39. | Joensuu H, Fletcher C, Dimitrijevic S, Silberman S, Roberts P, Demetri G. Management of malignant gastrointestinal stromal tumours. Lancet Oncol. 2002;3:655-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 395] [Article Influence: 17.2] [Reference Citation Analysis (0)] |