Published online Aug 15, 2004. doi: 10.3748/wjg.v10.i16.2373
Revised: September 24, 2003
Accepted: October 27, 2003
Published online: August 15, 2004
AIM: To investigate the intestinal barrier function damage induced by trauma and infection in rats.
METHODS: Experimental models of surgical trauma and infection were established in rats. Adult Sprague-Dawley rats were divided into 4 groups: control group (n = 8), EN group (n = 10), PN group (n = 9) and Sep group (n = 8). The rats in PN and Sep groups were made into PN models that received isonitrogenous, isocaloric and isovolumic TPN solution during the 7-d period. Rats in EN and Sep groups received laparotomy and cervical catheterization on day 1 and received lipopolysaccharide injection intraperitoneally on d 7. On the 7th day all the animals were gavaged with lactulose and mannitol to test the intestinal permeability. Twenty-four hours later samples were collected and examined.
RESULTS: The inflammatory responses became gradually aggravated from EN group to Sep group. The mucosal structure of small intestine was markedly impaired in PN and Sep groups. There was a low response in IgA level in Sep group when compared with that of EN group. Lipopolysaccharide injection also increased the nitric oxide levels in the plasma of the rats. The intestinal permeability and bacterial translocation increased significantly in Sep group compared with that of control group.
CONCLUSION: One wk of parenteral nutrition causes an atrophy of the intestinal mucosa and results in a moderate inflammatory reaction in the rats. Endotoxemia aggravats the inflammatory responses that caused by laparotomy plus TPN, increases the production of nitric oxide in the body, and damages the intestinal barrier function.
- Citation: Ding LA, Li JS, Li YS, Zhu NT, Liu FN, Tan L. Intestinal barrier damage caused by trauma and lipopolysaccharide. World J Gastroenterol 2004; 10(16): 2373-2378
- URL: https://www.wjgnet.com/1007-9327/full/v10/i16/2373.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i16.2373
Severe infection is still the main cause of death in critical illnesses such as severe trauma, burns and in massive use of immunosuppressive medicines. The endotoxin produced by G-bacilli is the important factor for the impairment of tissues and cells of the body. It could damage the systemic immunity, impair the intestinal barrier function, increase the mucosal permeability, and hence cause enterogenous infection, a phenomenon known as bacteria translocation[1,2]. The impairments aggravate the primary infection, which might cause multiple organ dysfunction syndrome (MODS), and even death. The alterations in pathophysiology and immunology when endotoxemia occurs, are very important for us to evaluate the impaired gut barrier function.
In routine clinical practice, some patients would complicate with infections after operation and trauma. Most of them can be cured, but a few of these infections would last for a longer time. Based on the consideration of the latter being a phenomenon of bacterial translocation as a result of the damage of intestinal barrier function, we conducted this animal experiment so as to investigate if there were gut barrier damage and bacterial translocation in such instances.
Adult, healthy male Sprague-Dawley rats, with body mass of 150-180 g (supplied by Shanghai Experimental Animal Center, Chinese Academy of Sciences) were used. The rats were fed for over 1 wk in our laboratory for adaptation and then put into metabolic cages for 5-7 d. The temperature in the animal rooms was 17-21°C with proper humidity (about 60%) and illumination of 12 h/d (6:00-18:00). During the adaptation period all the rats were fed with regular rat chow and tap water ad libitum. When the rat’s body mass reached 200-300 g, 33 rats were chosen randomly and divided into 4 groups: (1) control group (n = 8), which were fed rat chow and tap water freely; (2) EN group (n = 10), in which a central venous catheter was inserted into animals’ superior vena cava through right jugular vein under anaesthesia, and connected to the swivel apparatus. The rats also received laparotomy and were fed the chow freely; (3) PN group (n = 9), which were infused with a whole nutrients solution through a central venous catheter, and with drinking water ad libitum; (4) Sep group (n = 8) in which an exploratory laparotomy and central venous catheterization served as the trauma. After this, TPN was their sole nutrition source plus drinking water ad lib. On the 7th day 5 mg/kg of lipopolysaccharide was injected intraperitoneally for EN and SEP groups. PN and Sep Groups received isonitrogenous, isocaloric and isovolumic TPN solution during the 7-d period. All the protocols and procedures were approved by our University Committee of Animal Experiment Administration.
TPN ingredients About 11.4% compound amino acids injection (Novamin), 20% medium-long chain fat emulsion (Lipovenoes MCT), multivitamin mixture (Soluvit, Vitalipid) and a trace element mixture (Addamel) were purchased from Sino-Sweden and Fresenius Pharmaceutical Corp. LTD.
Chemicals and reagents Lipopolysaccharide (LPS, from E. coli, 055: B5) was purchased from Sigma Co.; NO Test Kit was purchased from Promega Co., USA. Immunohistochemistry (T cell subgroups measurement) reagents were purchased from Serotec Ltd., Germany and IgA, Bethyl Co., USA.
Operation procedures Under anaesthesia with 100 mg/kg of ketamin injected into the animals intraperitoneally, the TPN model was established and a rotary transfusion apparatus was used for TPN infusion[3]. For surgical trauma, after shaving the hair on the abdomen, an incision (about 4 cm in length) was made and the whole abdominal cavity was explored and examined for about 5 min from the epigastrium to the pelvic cavity. The incision was sutured in double layers with silk suture No. 1. The operation was done under aseptic conditions.
TPN solution The rats were put in the metabolic cages after surgical recovery. Each rat received 230 kcal/kg body mass of calories and 1.42 g nitrogen/kg each day in 50 mL of TPN solution. The ratio of glucose to lipid in this solution is 2:1, and nonprotein calorie to nitrogen (kcal/g), 137:1. Multivitamins, electrolytes, trace elements and 500 units of heparin were also included in the TPN solution. All the nutrient solutions were prepared under aseptic conditions daily and the infusion was done with an injecting micropump continuously and uniformly during 24 h each day. TPN infusion was started immediately after recovery from the laparotomy. On the first and last days of the experiment, each rat was given half of the total calories without any changes of other TPN ingredients.
Induction of endotoxemia On the 7th d of the experiment, 5 mg/kg of LPS in 5 mL of sterile distilled water was injected into the animals’ peritoneal cavity to cause a sepsis state.
Lactulose/mannitol solution gavage On the 7th d of the experiment, 66 mg of lactulose and 50 mg of mannitol dissolved in 2 mL of normal saline were gavaged. Twenty-four hour urine was collected, the volume recorded and 0.2 mL of mercury salicylosulfide added. Then 5 mL of the urine specimen was stored at -20 °C until measurement.
Twenty-four hours after gavaging with lactulose and mannitol and injecting endotoxin, 100 mg/kg of ketamine was injected intraperitoneally as an anesthetic. After the laparotomy was done, tissue and blood samples were collected and gross and laboratory examinations were performed.
Bacteriological test Blood 0.5 mL from the portal vein was drawn for culture. One gram of anterior lobe liver tissue and about 0.2-0.5 g mesenteric lymph nodes were excised. Each sample was put in a tissue homogenizer and 1.5 mL of normal saline was added before they were homogenized. The specimens were sent to a microbiological laboratory for aerobic culture and bacterial identification by morphological and bacteriological analyzer.
Bacterial culture (1) 10 µL homogenates of the lymph nodes and liver were separately taken and put on blood agar plates. Another 10 µL lymph nodes and liver homogenates were mixed with 10 mL saline for a dilution and the diluted samples were inoculated also on blood agar plates. (2) 0.5 mL of portal vein blood was inoculated into 4.5 mL of common broth for bacterial enrichment 16-18 h, then 20 µL of the enrichment solution was inoculated on blood agar plates. (3) The cultured media were put in a CO2 incubator at 35 °C for 24-48 h. If there was no bacterial growth, they would be regarded as negative; but if there was growth, it would be further identified.
Identification of bacteria First, Gram-stained smears were made to determine whether they were cocci or bacilli and G+ or G-. Second, identifications were made using bacteriological analyzer of Vitek-32 (Bio Merieux Vitek, Inc., USA). Gram-positive cocci were tested with GPI card, Gram-negative bacilli, with GNI card, and fungi, YBC card.
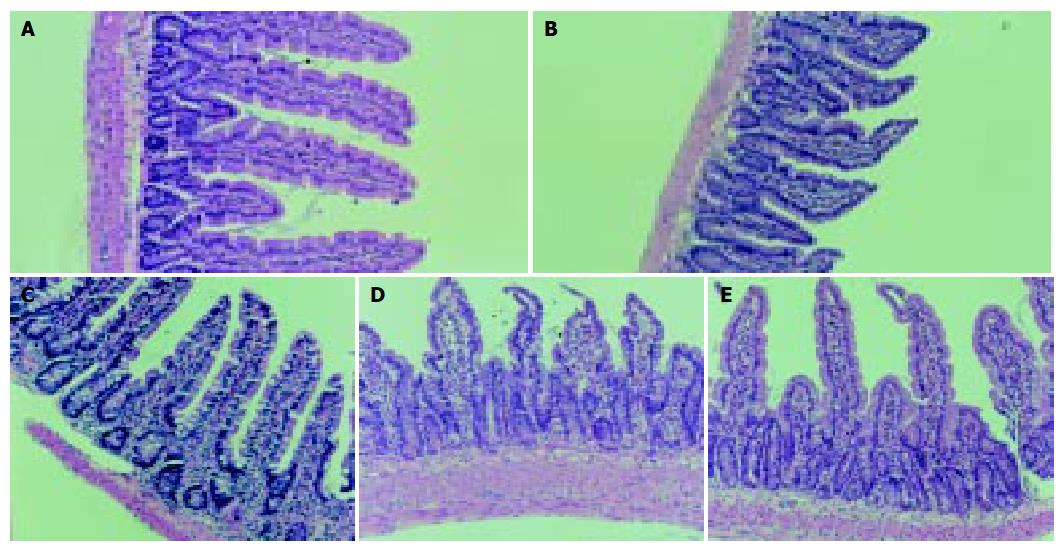
Preparation of small intestine specimens The whole small intestine below the Treitz ligament was excised and immediately placed on ice cold of 9 g/L saline. The intestine was opened longitudinally and the contents of the intestine were washed out with icy saline. Two cm of proximal jejunum and distal ileum were cut and put into a 40 g/L neutral formaldehyde solution promptly and sent to be examined histologically. About 5 cm intestine segments from the upper, middle and lower sections were resected and then the surfaces of the mucosa were dried with cotton swabs. The mucous membrane of the icy specimens was scraped, weighed and divided into two equal parts. They were immediately put into liquid nitrogen and then stored at -70 °C.
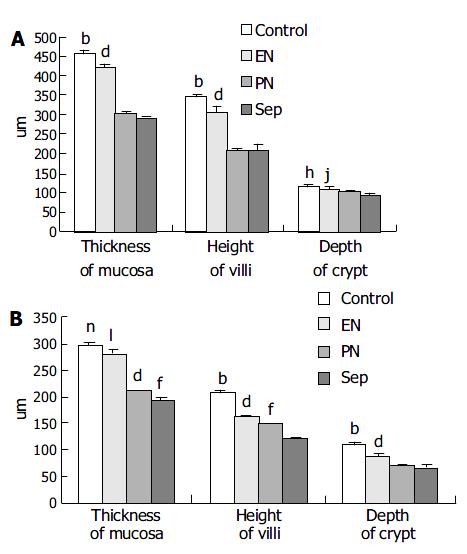
Histological examination of the intestinal mucosa Specimens were embedded in paraffin, 4 µm sections were cut and stained with H.E., analyzed with an HPIAS-1000 Multimedia Color Analysis System. Three low power (10×10) fields in each section were observed. The lengths of 5 villi, the depth of 5 crypts and the thickness of the mucosa at 5 sites were analyzed. The average value was calculated and documented. All the calculation was done double-blindly by two experienced pathologists.
Immunohistochemical analysis of T cell subpopulation[4] Specimens were embedded in paraffin, 4 µm sections were cut and stained with H.E., T cell subsets were examined by immunohistochemical SP method. The total T lymphotocyte and its positive stained subpopulations (CD3, CD4, CD8) were counted in 10 whole villi in each section. The ratios and rates were then calculated. All the calculation was done double-blindly by two experienced pathologists.
Blood sampling Three milliliter blood from the right cardiac ventricle was drawn and 1 250 u heparin added. The blood was centrifuged and the serum stored at -70 °C. Then the animals were sacrificed by exsanguination.
Lactulose/mannitol test The lactulose and mannitol concentrations in the preserved urine sample were measured by a high-performance liquid chromatograph (Waters Co., USA). The ion-exchange column used was purchased from Transgenomic Co., USA. The ratio between the two sugars was then calculated. The test was to measure the amount of excreted dual sugars in urine of 24 h. Because these two kinds of saccharide nearly neither metabolize nor synthesize in the body, amounts of the two sugars being excreted from urine reflect the “leaking” degree of the intestine, or permeable extent of the intestine. The molecular weight of these two saccharides is different. The test error can be reduced when using two sugars of different molecular weight than using one only for the test. If the ratio of the two sugars’ percentage in tested group was significantly bigger than that of normal control group, it could be concluded that the intestinal permeability in tested animals increased.
Determination of IgA The frozen samples of blood plasma and mucosa of the small intestine were melted to room temperature and 1 mL normal saline was then added to the melted 100 mg of mucosa before the homogenates were made, and then the concentration of IgA in their supernatants after centrifugation below 4 °C was measured. The results were shown as IgA µg/mL of blood plasma and IgA µg/g of small intestine mucosa.
Determination of NO The frozen samples of blood plasma and mucosa of the small intestine were melted to room temperature and concentration of NO in them was measured with the enzymatic method (Griess Reaction)[5] .The results were shown as NO µmol/L of blood plasma and NO µmol/g of small intestine mucosa.
All the values were expressed as the mean ± SE. One-way ANOVA was used to check the differences between groups. Chi-square test was used to check the differences of bacterial translocation rates between groups. When P was less than 0.05, the difference was considered statistically significant. The degree of correlation was described using Pearson correlation coefficient. Software SPSS10.0 was used in all statistical tests.
All the rats except those in the normal group appeared to have symptoms in different degrees, such as lethargy, idleness, ruffling of hair, stop drinking and grossly concentrated urine. Some rats appeared very slow in their responses to sound stimulations and also had diarrhea. Their eyes appeared glazed with crusting exudates. These symptoms were prominent during the 5 to 15 h following LPS injection. There was no statistical difference in mortality within 24 h after LPS injection between EN and Sep groups.
The total number of leukocytes and the number of neutrophils in PN group [(14.97 ± 2.704) × 10 9/L and (6.82 ± 2.254) × 10 9/L] was more than those of control group [(7.59 ± 0.379) × 10 9/L and (2.34 ± 0.568) × 10 9/L]. On the contrary, Sep group rats showed a low response in cell count [(8.9 ± 0.82) × 10 9 and (4.35 ± 0.92) × 10 9/L]. Platelet count in groups of EN, PN and Sep [(586.5 ± 65.03) × 10 9, (483.33 ± 94.62) × 10 9 and (277.5 ± 44.87) × 10 9/L respectively] decreased markedly as compared with that of control group [(928.7 ± 33.89) × 10 9/L].
At the beginning of the experiment there was no difference in the body mass of the animals. At the end of the experiment, the body mass increase was 32.38 ± 3.39 g in the control group. The body mass of other three groups decreased; it was 33.88 ± 3.19 g of loss in Sep group, the greatest among them.
The degree of damage of the villi and crypts and the thinning of the mucous membrane in jejunum and ileum were most significant in Sep group among the animals (Figure 1, Figure 2).
Concentrations of NO in blood plasma increased significantly after LPS injection (groups EN and SEP), whereas it was not evident in mucous membrane of small intestine (Table 1).
IgA level was highest in blood plasma and intestinal mucosa in EN group among the animals (Table 2).
There was no difference in CD3 population among the groups but CD4/CD8 ratio in EN and PN groups was higher than that of control group. There was no significant difference in ratio of CD4 to CD8 between Sep group and control group.
The L/M ratio showed a significant increment in all rats except those in control group animals (Table 3).
The results of bacterial culture were labeled as positive when the CFU found per gram of tissue (or mL of blood) was more than or equaled to 103[6]. In Sep group the rate of bacterial translocation was the highest. The logarithm of the number of translocated bacteria correlated positively with the rate of bacterial translocation (Table 4). The bacteria translocated, in order of frequency, were proteus, E. coli, enterococcus and other Gram-negative bacteria. One or two, even three bacterial groups were usually recovered from the same organ when translocation was present.
Recent studies have shown that the gastrointestinal tract is not only an organ for nutrient digestion and absorption, but also an organ for systemic immunity and at the same time performs a barrier function. It prevents bacteria and endotoxin in the lumen of the GI tract from entering the blood circulation (bacterial/endotoxin translocation). At the time when body and/ or organ tissues are injured by trauma, burns, infection, ischemia/reperfusion and surgical operation strikes, the intestinal barrier malfunction occurs and enterogenous infection ensues. Thus, it aggravates the original illness and makes it persistent, causing multiple organ dysfunction syndrome (MODS) and even death[1,2,6-9]. Based on these, Wilmore named the GI tract as “a central organ after surgical stress”[3].
Most infections that take place in abdominal surgery are commonly caused by G- bacilli or polyinfection, which brings harm to body through endotoxin. Endotoxin (LPS) plays a very important role during stress. In vivo and in vitro studies in animals and humans indicated that LPS could cause damage to the intestinal barrier function and cause bacterial translocation[1,3,6]. The aim of the experiment was to mimic the clinical cases above. In the experiment, we had laparotomy plus parenteral nutrition as trauma. Sick manifestations emerged after a few days’ parenteral nutrition. One wk parenteral nutrition was obviously an inflammatory stimulus to rats. Laparotomy was a minor injury, whereas it was an intermediate degree of impairment when administering laparotomy plus 1-wk parenteral nutrition to rats. Clinical manifestations in rats confirmed the point of view. We injected lipopolysaccharide to rats intraperitoneally after 1-wk parenteral nutrition so as to mimic infested clinical situations after trauma. There were two main harmful stimuli, parenteral nutrition and LPS injection, included in our experiment. We have had another two control groups, the group of parenteral nutrition (PN) and the group of LPS injection with enteral feeding (EN) except the normal control one so as to investigate and compare the damage degree of different injury stimuli to gut barrier.
Investigations have reported the relation of TPN with bacterial translocation. Qin and his colleagues[10] discovered that parenteral feeding in a 7-d period in experimental pancreatitis of dogs caused a significant damage of intestinal mucosa and bacterial translocation (BT) when compared with that of given isonitrogenous and isocaloric enteral feeding. Similar results were reported by other scholars[11,12]. A study even revealed that 2-d TPN resulted in BT in SD rats[13]. In their investigation patients underwent thoracic esophagectomy, Takagi et al[14] presented that in patients receiving TPN from 1 wk before operation to 2 wk after operation, serum levels of IL-6, IL-10 and endotoxin were higher than that of receiving isonitrogenous and isocaloric TEN. The result suggested that TPN could increase the endotoxin translocation from the bowel.
Our investigation achieved similar results and showed that feeding rats with TPN for 1 wk led to a remarkable atrophy of the intestinal mucous membrane; hence an increase in intestinal permeability and bacterial translocation ensued. The rats were in a state of systemic inflammatory response, which was shown by clinical manifestations of the animals and analysis of their blood cells. It is known that the cause of increment in intestinal permeability in animals fed with TPN is mainly the atrophy of the intestinal mucous membrane[15,16]. Parenteral nutrition could meet the needs of other organs and tissues of the body, but not that of the intestinal mucosa. Seventy percent of the nutrients that intestinal epithelia need is absorbed directly from the intestinal lumen by mucosa cells[17]. Enteral feeding could prevent the intestinal mucous membrane from atrophy and avoid increment of intestinal permeability[10-12] . Likewise, it could also prevent gut associated lymphatic tissue (GULT) from atrophy, which could increase the intestinal mucosal immunity[16,17]. It could be seen from our investigation that the damage of intestinal mucous membrane in EN rats was greatly attenuated. Enteral feeding led to a better immune reaction in these rats than others. The increase of gut permeability was avoided and the rate of bacterial translocation alleviated. It was shown from the analysis of complete blood cell count in the rats that the total number of leukocytes and the number of neutrophils increased following the stimulus of TPN in PN group, whereas there was a low reaction in Sep group, which suffered from more severe stress injury than PN group. The falling of platelet count may be related to a greater depletion of platelets that was caused by an intravascular coagulation due to the systemic inflammatory reaction[1,18].
A series of symptoms appeared after injecting LPS into laparotomised TPN-rats in our experiment: lethargy, idleness, ruffling of hair, stopping drinking and grossly concentrated urine etc. Some rats appeared to have very low reactions to sound and other stimulations and also had diarrhea. Some eyes appeared glazed with crusting exudates. These symptoms were prominent during the 5 to 15 h following LPS injection, and the menifestations were alleviated gradually thereafter. These were in accordance with other reports[1,3,6,18-20]. No statistical difference was found in the mortality within 24 h after LPS injection between the two groups (there were 1 and 3 deaths for EN and Sep groups respectively).
The animals in Sep group responded acutely after being injected with LPS intraperitoneally. LPS aggravated the impairment of the intestinal mucosal barrier and bacterial translocation that was caused by TPN. The aggravation of impairment of the mucosal barrier function might be related to nitric oxide (NO), which was shown by an increased NO level in blood plasma after LPS injection. Some scholars considered that high output of NO after surgical stress could damage the oxygenation metabolism in mitochondria of the intestinal epithelia[3,21].
The increment of intestinal permeability caused by endotoxin is very complicated. It may relate to many inflammatory mediators such as cytokines, vasoactive amines and oxygen free radicals[22,23]. Among them, NO, one of the main oxygen free radicals in the body, is an important inflammatory mediator causing impairment of the intestinal barrier[3,18,24]. Generally, the body could only secrete small amounts of NO under the effects of constructive NO synthase (cNOS) and endothelial NO synthase (eNOS). This low level of NO has protective effects on the body[20], whereas a higher concentration of NO being produced under the effect of inducible NO synthase (iNOS) when the body suffered from harmful stimulations, would impair the functions of cells and tissues of the body, including the intestinal barrier function. It was concluded from a great number of studies that a lower NO level was beneficial and a higher NO level was harmful to the body[3,23,25], and using NO inhibitor when stress occurred could reduce the damage of gut barrier[26,27]. The results from our investigation were in accordance with this hypothesis.
The intestinal immunity is very important in maintaining intestinal barrier function. It is known that the digestive tract is the largest immune organ in the human body. About 80% of humoral immunity and 50% of cellular immunity locate in the digestive tract[25,28]. Intestinal mucosal IgA is the first defense line of the intestinal barrier. It has an important function in preventing bacterial adherence and translocation from intestinal lumens[21]. The IgA secretion in our EN group increased after LPS stimulation, but it had a low reaction in PN group when compared with that of EN group. The ratio of CD4 to CD8 in mucous membrane of small intestine in Sep group was lower than that in groups EN and PN, reflecting a low cellular immune response. This is in accordance with other reports that LPS could stimulate the increment of suppressive T lymphocytes[22]. IgA level in blood plasma is positively correlated with that in the intestinal mucosa, and this accords with a previous report[29]. Our results indicated that the increase of bacterial translocation was not only caused by an increased intestinal permeability, but also by an impairment of the whole intestinal barrier function[16,30]. The kinds and groups of translocated bacteria and the phenomenon that there were 1 to 3 kinds of translocated bacteria in the same organ found in our experiment were in accordance with other reports[3,31].
In summary, one week of parenteral nutrition caused an extreme atrophy of intestinal mucosa and an impairment of intestinal barrier function in SD rats. LPS aggravated this damage and also damaged the systemic immunity of the animals. The aggravation was related to the increased NO produced by the stimulation of LPS.
We would like to thank Professor Hai-Feng Shao and Professor Gen-Bao Xu for their technical guidance and advice; we are grateful to the staff of the Animal Laboratory and the Institute of General Surgery of Nanjing University Medical School for their generous support and assistance to our study.
Edited by Zhang JZ and Chen WW Proofread by Zhu LH and Xu FM
| 1. | van Deventer SJ, ten Cate JW, Tytgat GN. Intestinal endotoxemia. Clinical significance. Gastroenterology. 1988;94:825-831. [PubMed] |
| 2. | Yu P, Martin CM. Increased gut permeability and bacterial translocation in Pseudomonas pneumonia-induced sepsis. Crit Care Med. 2000;28:2573-2577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Dickinson E, Tuncer R, Nadler E, Boyle P, Alber S, Watkins S, Ford H. NOX, a novel nitric oxide scavenger, reduces bacterial translocation in rats after endotoxin challenge. Am J Physiol. 1999;277:G1281-G1287. [PubMed] |
| 4. | Whiteland JL, Nicholls SM, Shimeld C, Easty DL, Williams NA, Hill TJ. Immunohistochemical detection of T-cell subsets and other leukocytes in paraffin-embedded rat and mouse tissues with monoclonal antibodies. J Histochem Cytochem. 1995;43:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8653] [Cited by in RCA: 9032] [Article Influence: 210.0] [Reference Citation Analysis (0)] |
| 6. | O'Dwyer ST, Michie HR, Ziegler TR, Revhaug A, Smith RJ, Wilmore DW. A single dose of endotoxin increases intestinal permeability in healthy humans. Arch Surg. 1988;123:1459-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 187] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Wilmore DW, Smith RJ, O'Dwyer ST, Jacobs DO, Ziegler TR, Wang XD. The gut: a central organ after surgical stress. Surgery. 1988;104:917-923. [PubMed] |
| 8. | Berg RD. Bacterial translocation from the gastrointestinal tract. Trends Microbiol. 1995;3:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 291] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 9. | Swank GM, Deitch EA. Role of the gut in multiple organ failure: bacterial translocation and permeability changes. World J Surg. 1996;20:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 424] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 10. | Qin HL, Su ZD, Hu LG, Ding ZX, Lin QT. Effect of early intrajejunal nutrition on pancreatic pathological features and gut barrier function in dogs with acute pancreatitis. Clin Nutr. 2002;21:469-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Mosenthal AC, Xu D, Deitch EA. Elemental and intravenous total parenteral nutrition diet-induced gut barrier failure is intestinal site specific and can be prevented by feeding nonfermentable fiber. Crit Care Med. 2002;30:396-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Li J, Langkamp-Henken B, Suzuki K, Stahlgren LH. Glutamine prevents parenteral nutrition-induced increases in intestinal permeability. JPEN J Parenter Enteral Nutr. 1994;18:303-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Pscheidl E, Schywalsky M, Tschaikowsky K, Böke-Pröls T. Fish oil-supplemented parenteral diets normalize splanchnic blood flow and improve killing of translocated bacteria in a low-dose endotoxin rat model. Crit Care Med. 2000;28:1489-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Takagi K, Yamamori H, Toyoda Y, Nakajima N, Tashiro T. Modulating effects of the feeding route on stress response and endotoxin translocation in severely stressed patients receiving thoracic esophagectomy. Nutrition. 2000;16:355-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Sugiura T, Tashiro T, Yamamori H, Takagi K, Hayashi N, Itabashi T, Toyoda Y, Sano W, Nitta H, Hirano J. Effects of total parenteral nutrition on endotoxin translocation and extent of the stress response in burned rats. Nutrition. 1999;15:570-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | MacFie J. Enteral versus parenteral nutrition: the significance of bacterial translocation and gut-barrier function. Nutrition. 2000;16:606-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | van der Hulst RR, von Meyenfeldt MF, van Kreel BK, Thunnissen FB, Brummer RJ, Arends JW, Soeters PB. Gut permeability, intestinal morphology, and nutritional depletion. Nutrition. 1998;14:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 73] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Fish RE, Spitzer JA. Continuous infusion of endotoxin from an osmotic pump in the conscious, unrestrained rat: a unique model of chronic endotoxemia. Circ Shock. 1984;12:135-149. [PubMed] |
| 19. | Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res. 1980;29:189-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1078] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 20. | Schmidt H, Secchi A, Wellmann R, Bach A, Böhrer H, Gebhard MM, Martin E. Effect of endotoxemia on intestinal villus microcirculation in rats. J Surg Res. 1996;61:521-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Unno N, Wang H, Menconi MJ, Tytgat SH, Larkin V, Smith M, Morin MJ, Chavez A, Hodin RA, Fink MP. Inhibition of inducible nitric oxide synthase ameliorates endotoxin-induced gut mucosal barrier dysfunction in rats. Gastroenterology. 1997;113:1246-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 216] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 22. | Marshall JC, Christou NV, Meakins JL. Small-bowel bacterial overgrowth and systemic immunosuppression in experimental peritonitis. Surgery. 1988;104:404-411. [PubMed] |
| 23. | Mishima S, Xu D, Deitch EA. Increase in endotoxin-induced mucosal permeability is related to increased nitric oxide synthase activity using the Ussing chamber. Crit Care Med. 1999;27:880-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Forsythe RM, Xu DZ, Lu Q, Deitch EA. Lipopolysaccharide-induced enterocyte-derived nitric oxide induces intestinal monolayer permeability in an autocrine fashion. Shock. 2002;17:180-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Nadler EP, Ford HR. Regulation of bacterial translocation by nitric oxide. Pediatr Surg Int. 2000;16:165-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Hsu CM, Liu CH, Chen LW. Nitric oxide synthase inhibitor ameliorates oral total parenteral nutrition-induced barrier dysfunction. Shock. 2000;13:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Deitch EA, Shorshtein A, Houghton J, Lu Q, Xu D. Inducible nitric oxide synthase knockout mice are resistant to diet-induced loss of gut barrier function and intestinal injury. J Gastrointest Surg. 2002;6:599-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Hulsewé KW, van Acker BA, von Meyenfeldt MF, Soeters PB. Nutritional depletion and dietary manipulation: effects on the immune response. World J Surg. 1999;23:536-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Brandtzaeg P, Halstensen TS, Kett K, Krajci P, Kvale D, Rognum TO, Scott H, Sollid LM. Immunobiology and immunopathology of human gut mucosa: humoral immunity and intraepithelial lymphocytes. Gastroenterology. 1989;97:1562-1584. [PubMed] |
| 30. | Deitch EA, Ma WJ, Ma L, Berg RD, Specian RD. Protein malnutrition predisposes to inflammatory-induced gut-origin septic states. Ann Surg. 1990;211:560-567; discussion 560-567;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Naaber P, Smidt I, Tamme K, Liigant A, Tapfer H, Mikelsaar M, Talvik R. Translocation of indigenous microflora in an experimental model of sepsis. J Med Microbiol. 2000;49:431-439. [PubMed] |