INTRODUCTION
New ultrasound contrast consisting of perfluorocarbon microbubbles could enhance grey scale images of parenchymal organs. It provided a new method in diagnosis[1-10]. The development of new ultrasound contrast agent (UCA) has become one of the most promising fields in ultrasound medicine. So far, several UCAs, like Optison[11,12] and Definity[13], have been approved for treatment and become commercially available; many others are still in clinical trials[14-16]. Based on the chemical components of bubble film, UCAs could be divided into four different types: Surfactants[17-19], human albumin[20,21], polymer[22] and lipids[23-25]. The lipids are superior to the others in many aspects: no risk of blood transmitted infection, excellent stability and some tissue-specific targeting, like the reticuloendothelial system. This study was aimed to investigate the effectiveness, reproducibility of a new lipid contrast agent in the enhancement of abdominal parenchymal organs. All the protocols were approved by IRB (Internal Review Board) of Xinqiao Hospital.
MATERIALS AND METHODS
Preparation and analysis of lipid-coated microbubble agent
Liposome, which consists of two kinds of phospholipids and polyethylene glycol, was prepared by lyophilization. Then the lipids were rehydrated with certain media, like glucose and deionized water. The resultant suspension was sonicated by an ultrasound sonicator (YJ 92-II Xinzhi Corp. Hang-zhou, Zhejiang Province). The perfluoropropane was introduced into the suspension during sonication, then it was kept still, until it was separated into two layers, the upper layer was still in white colloid whereas the lower layer was slowly defecated. Then the upper layer of the agent was extracted and analyzed. To acquire size distribution data of the new contrast agent, the milky microbubbles suspension was analyzed by Sysmex KX-21 (Sysmex Corporation, Japan). The surface electric potential and pH of UCA were measured by Zeta 3000 (Malvern Ltd., United Kingdom).
Animal models
Ten healthy rabbits, weighing 2.0-2.2 kg, were enrolled in this study. The body hair at experimental region was removed for liver and renal scan. Rabbits were anesthetized by intramuscular injection of “Xu Mian Xing” (mainly consisted of haloperidol and made by Changchun University of Agriculture and Prologue) at the dose of 0.15 mL/kg bm, and the intravenous infusion was set up through ear veins. Lipid contrast agent was injected at 0.01 mL/kg bm, and followed by 1 mL saline flush.
Settings of ultrasound equipment
A Siemens Sequoia 512 (Siemens Acuson Co., Mountain View, California) ultrasound system was used in this study. The second harmonic imaging of 6L3 probe at 3.0/6.0 MHz was used. The mechanic index and output power were set at 0.11 and -24 dB, respectively. All the other parameters, like gain, depth, TGC, compress and focus, were kept constant during experiment. Experimental images were digitally recorded. Overall, baseline and 60 min contrast images were acquired. All images were transformed from original DICOM files into JPEG format by Viewpro (Acuson Co.). Grey scale was calculated by histogram in Adobe Photoshop 6.0 (Adobe Co.). Sample area used in the histogram had 1088 pixels in an ellipse. Time-density curve was generated based on the mean pixel grey scale of hepatic parenchyma and renal cortex.
Pathological examination
All animals were sacrificed by intravenous injection of 10% potassium chloride. The liver, kidney and lung tissues were autopsied and fixed in 40 g/L formaldehyde for H&E stain. All tissue slides were reviewed by pathologists.
Statistical analysis
The grey scale data acquired from liver, inferior vena cava and renal cortex were expressed as mean ± SD. Pre- and post-contrast of liver’s and kidney’s grey scale data were compared by paired two-tailed Student’s t test in SPSS 8.0 program. P critical value less than 0.05 was considered to be statistically significant.
RESULTS
Contrast agent
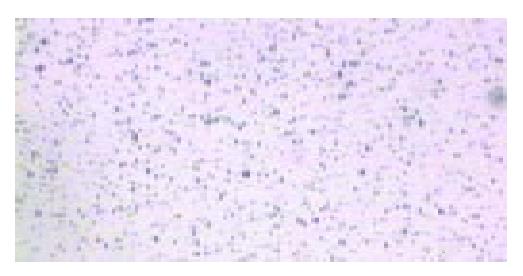
The visual appearance of the newly made liposome contrast agent was an opaque milky suspension, and it was slowly delaminated. The microbubble concentration was (7-8) × 10 9/mL, and the size distribution was 2 to 10 µm. About 90% of the microbubbles were less than 8 µm (Figure 1). The surface electric potential was -71.2 mV and the pH was 6.42.
Figure 1 Microbubbles in 400-fold diluted saline (original magnification: × 100).
Grey scale enhancement of liver and kidney
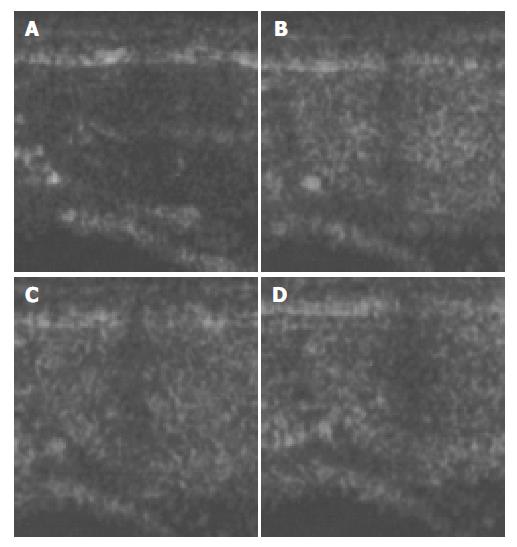
The images of both hepatic parenchyma and renal cortex were visually significantly enhanced after intravenous injection of the contrast agent (Figure 2, Figure 3, Figure 4, Figure 5). Time-intensity curves of both liver and cortex were elevated and kept at a high level. The grey scale of liver parenchyma (61.2 ± 3.1) 30 s after contrast injection was significantly higher than that of baseline image (38.0 ± 3.0, P < 0.05). At the time point of 180 s after injection, the grey scale of liver parenchyma reached 83.2 ± 1.8 which was significantly higher than baseline (P < 0.01). And 54 min after the injection, the liver enhancement remained at 57.8 ± 1.4. One hour after contrast injection, the grey scale dropped to 45.2 ± 1.0, which had no statistical significance. Similar results were observed in the contrast enhancement of renal cortex, and the grey scales were 18.1 ± 3.8 at baseline, 48.8 ± 3.3 ( P < 0.01) 10 s after injection, 27.4 ± 4.1 ( P < 0.01) 56 min after injection, 26.1 ± 3.9 60 min after injection (P < 0.05). These results confirmed that this lipid microbubbles contrast agent could effectively enhance hepatic parenchyma and renal cortex at a relatively long time.
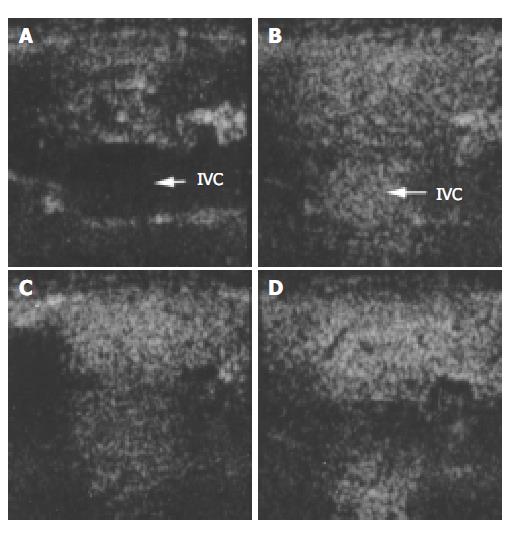
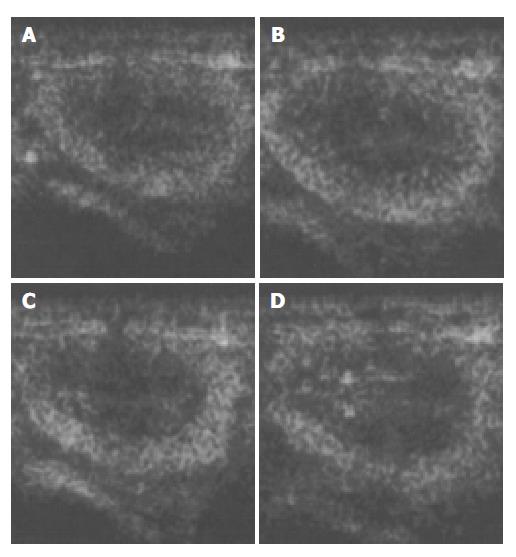
Figure 2 Ultrasound images of liver parenchyma and inferior vena cava (IVC) before and after the injection of microbubbles.
A: before injection; B: 30 s after injection; C: 1 min after injection; D: 2 min after injection.
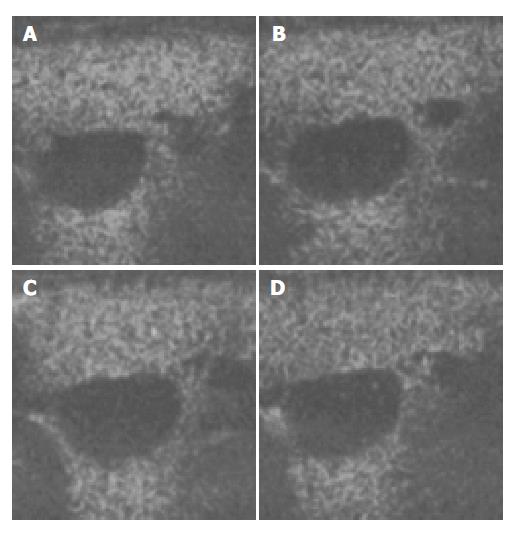
Figure 3 Ultrasound images of liver parenchyma and inferior vena cava (IVC) before and after the injection of microbubbles.
A: 15 min after injection; B: 25 min after injection; C: 30 min after the injection; D: 40 min after the injection.
Figure 4 Ultrasound images of renal cortex before and after the injection of microbubbles.
A: before injection; B: 30 s after injection; C: 1 min after injection; D: 2 min after injection.
Figure 5 Ultrasound images of the renal cortex after injection of microbubbles.
A: 5 min after injection; B: 10 min after injection; C: 30 min after injection; D: 40 min after injection.
At inferior vena cava (IVC), the time-intensity curve was different from that of hepatic parenchyma and renal cortex. The grey scale of IVC significantly rose to 52.5 ± 2.9 20 s after contrast injection which was higher compared with baseline (5.8 ± 1.6). The significant enhancement lasted only 120 s after injection, then diminished gradually and disappeared at 240 s after contrast injection. IVC contrast intensity dropped back to baseline 27 min after injection (17.0 ± 5.2).
Pathological results
All tissues from liver, lung, and kidney were normal in histology, and there was no sign of air embolism or infarction spot.
DISCUSSION
Lipid-coated microbubbles were mainly composed of several biocompatible phospholipids that had excellent stability and non-bioactive components. The lipids had been widely used in the preparation of ultrasound contrast agent, liposome and other drugs. In this study, a new ultrasound contrast agent based on lipids was prepared and showed excellent stability in the enhancement of hepatic parenchyma and renal cortex at a minimal dose of 0.01 mL/kg bm. Grey scale enhancement could last more than 50 min from both visual assessment and quantitative statistical analysis. This enhancement was longer than some previous reports, which were about 5-15 min[19,25,26]. Also, the time-intensity curves of hepatic parenchyma and renal cortex were quite different from that of inferior vena cava. These microbubbles tended to be stable in parenchyma, rather than in circulation, as the time-density curve from IVC showed a 200-s wash-in and wash-out enhancement.
According to some reports[27,28] and our experiment[29], we assumed that the prolonged enhancement was due to the uptake of microbubbles by the reticuloendothelial system in liver. But this could not explain the prolonged enhancement in the kidney, which is not rich of reticuloendothelial system. There might be some other reasons. Since the peak contrast intensities of liver parenchyma and cortex appeared much later than that of inferior vena cava, they were 12 min, 6 min and 30 s, respectively, it indicated that after the peak contrast in circulation there was another peak contrast in solid organs, and it was presumed to be the accumulation of microbubbles in hepatic sinusoids or capillary bed in cortex. As we know, hepatic parenchyma is consisted of hepatic sinusoids and renal cortex is mostly consisted of glomeruli. They are both rich in capillaries. Meanwhile, the blood flow in hepatic sinusoids and glomeruli is much slower than that in inferior vena cava. So, the microbubbles could easily be accumulated in capillary bed, which resulted in the prolonged enhancement of liver and kidney.
As IVC grey scale at 27 min postinjection was lower than that before injection, the hepatic parenchyma might block ultrasound transmission to IVC by condensed bubble’s attenuation, and this could produce visually lower IVC echo.
In this study, the new lipids ultrasound contrast agent was long-term stable in enhancement of liver parenchyma and renal cortex. The mean size of the microbubbles was less than 8 µm, which was smaller than that of red blood cell, and thereby it was safe for intravenous injection. Pathologically, all the tissues from liver, lung, and kidney were normal in histology, and there were no signs of air embolism or infarction spot.
In comparison with other lipid-coated microbubbles[30], this contrast agent had a higher bubble concentration and a longer stability in parenchymal organs. The reason for this might attribute to the reconstituted membrane of the microbubbles. Polyethylene glycol could protect phagocytosis from Kupffer cell, and it could exist in hepatic sinusoid longer. Tween 80, a kind of surfactant, which has never been used in other lipid microbubbles contrast agent, may increase the bubbles or microbubbles concentration.
Although this new lipid-based contrast agent exhibited a prolonged enhancement, the true reason has not been clarified. Further studies are needed to investigate the mechanism of long-lasting enhancement.