INTRODUCTION
RNA interference (RNAi) is a potent gene silencing mechanism conserved in all eukaryotes, in which double-stranded RNAs suppress the expression of cognate genes inducing degradation of mRNAs or blocking mRNA translation[1-3]. The physiological functions of RNAi are not very clear as yet, but it has been used successively as a potent method of gene knockdown in study of gene function and in experimental treatment of some diseases[4-19]. In mammalian cells, double-stranded RNAs inducing RNAi must be short in length (< 30 bp) so that they would not activate nonspecific interferon reactions. These short interfering RNAs (siRNAs) can be produced by four different ways: chemical synthesis, in vitro transcription, enzymatic digestion of dsRNAs and transfection of DNA vectors encoding siRNAs. Among the four ways, transfection of DNA vectors has some advantages such as low cost, lasting expression of siRNA, easiness of preparation, which make it the preferential method when siRNAs are used in treatment of diseases.
Hepatitis B virus (HBV) is a small enveloped DNA virus which belongs to hepadnaviridae. Human beings are HBV’s natural host, though some primates can be infected in laboratory. Liver is the primary target organ of infection and persistent infection of HBV usually leads to severe diseases, such as chronic hepatitis, cirrhosis and hepatocellular carcinoma. Current treatment regimens for chronic HBV infection, namely administration of interferon-γ, lamivudine, adefovir, or different combinations of these drugs have only limited long-term efficacy and many adverse effects or drug resistance[20,21]. Considering the huge population infected by HBV (more than 350 million people are infected by HBV around the world), the exploration of novel treatment strategies is both necessary and urgent. Many ingenious treatment strategies have been tested for inhibition of HBV replication, such as antisense nucleotides, ribozymes, intracellular antibodies, and targeted ribonucleases[22], and all of them have been demonstrated to inhibit HBV replication to various degrees.
To explore RNAi as a novel alternative strategy for treating HBV, we constructed a DNA vector which can express siRNAs in mammalian cells, and then the vector was used to express in 2.2.15 cells a siRNA against HBV to study its effect on HBV replication.
MATERIALS AND METHODS
Cell culture
Human hepatoblastoma Hep G2 cell line 2.2.15 cells stably transfected by HBV genome, were cultured in Dulbecco’s modified Eagle’s medium (DMEM, from Gibco Life Technologies, Grand Island, NY) supplemented with 100 mL/L fetal calf serum (Sijiqing Biotech Company, Hangzhou, China).
Construction of mammalian siRNA expression vector-pUC18U6
Human genomic DNA was extracted from HepG2 cells with DNeasy™ tissue kit (Qiagen, Germany) according to the manufacturer’s protocol. Purified human genomic DNA was used as the template in PCR to obtain human U6 promoter. The sequences of the primers are as follows: Forward primer: 5’GCGATCTAGAAAGGTCGGGCAGGAAGAG3’, reverse primer: 5’GCGAGGTACCGGTGTTTCGTCCTTTCCACAAG3’.
The underlined bases in forward and reverse primers have recognition sites for Xba I and Kpn I, respectively. The condition of PCR was at 94 °C for 5 min, then 35 cycles at 94 °C for 30 s, at 60 °C for 30 s, and at 72 °C for 1.5 min followed by extension at 72 °C for 7 min. The PCR product was analyzed with 100 g/L agarose gel electrophoresis and then purified by PCR fragment recovery kit as recommended by the manufacturer (Takara, Dalian, China). The purified PCR product was digested by Xba I and Kpn I, ligated with pUC18, and transformed into competent E. coli. The recombinant plasmid was then purified from transformed E. coli, and verified by Xba I/Kpn I digestion analysis and DNA sequencing.
Construction of HBV-siRNA expression vector-pUC18U6HBVsir
Two oligodeoxyribonucleotides encoding shRNA against HBV were synthesized by Bioasia Company (Shanghai) and their sequences are as follows: Oligo 1:5’CAGGCTTTCACTTTCTCGCTCGAGCGAGAAAGTGAAAGCCTGCTTTTTTG3’, Oligo2:5’AATTCAAAAAAGCAGGCTTTCACTTTCTCGCTCGAGCGAGAAAGTGAAAGCCTG3’.
The underlined bases are spacer region and recognition site for Xho I. The target of shRNA was 1086-1103 nt of HBV genome. To clone the two oligodeoxyribonucleotides, downstream of U6 promoter into pUC18U6, 400 nmol/L for each of the two oligodeoxyribonucleotides was mixed, heated at 100 °C for 5 min, and cooled gradually to room temperature to anneal. pUC18U6 was digested with Kpn I, blunt-ended with T4 DNA polymerase, then digested with EcoR I, purified and ligated with the annealed oligodeoxyribonucleotides. The ligation mixture was transformed into competent E. coli. The recombinant plasmid was then purified from transformed E. coli, and verified by Hind III/Xho I digestion analysis. A control vector, pUC18U6GFPsir, which expressed a siRNA against green fluorescent protein (GFP), was constructed with the same methods and the sequences of the two oligodeoxyribonucleotides encoding the siRNA were synthesized as previously described[23]: Oligo1:5’GGCGATGCCACCTACGGCAAGCTCGAGCTTGCCGTAGGTGGCATCGCCCCTTTTTTG3’,Oligo2:5’AATTCAAAAAAGGGCGATGCCACCTACGGCAAGCTCGAGCTTGCCGTAGGTGGCATCGCC3’.
The underlined bases are spacer region and recognition site for Xho I.
Transfection
Twenty-four hours before transfection, 2.2.15 cells were seeded into the culture plate at a density of 2 × 108/L. LipofectamineTM 2000 reagent (Gibco Life Technologies, Grand Island, NY) was used for the transfection of 2.2.15 cells by pUC18U6HBVsir, pUC18U6, pUC18U6GFPsir, or mock solution (DMEM plus LipofectamineTM 2000 reagent containing no plasmid) according to the manufacturer’s protocol.
Quantification of HBsAg in supernatant of cell culture
Forty-eight hours after the transfection, the supernatant of the cell culture was collected, and HBsAg in the supernatant was quantified using the solid phase radio-immunoassay kit for quantification of HBsAg (Beimian Dongya Biotech Institute, Beijing) according to the manufacturer’s protocol. Briefly, 200 μL of the supernatant was incubated with a plastic tube coated by anti-HBs antibody at 45 °C for 1.5 h. After the tube was thoroughly washed with deionized water, 200 μL of 125I-labelled secondary antibody was added and incubated at 45 °C for 2 h. The tube was thoroughly washed, and cpm was measured. The concentration of HBsAg of each sample was calculated by comparing the cpm of each sample with a standard curve.
Statistical analysis
One-way ANOVA was used for data analysis. Differences were considered significant when P < 0.05.
RESULTS
Construction of pUC18U6 and pUC18U6HBVsir
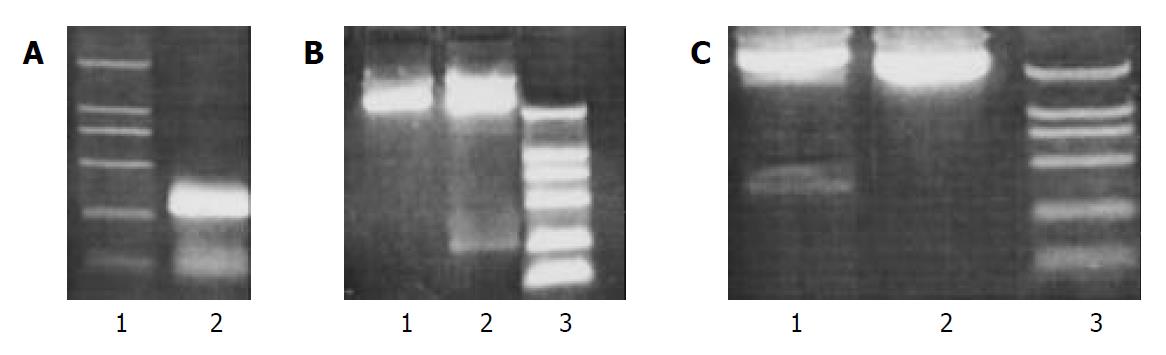
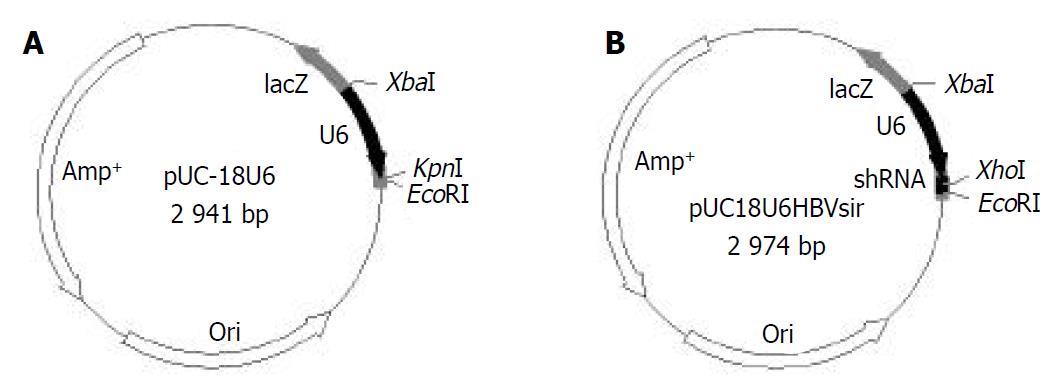
Agarose gel analysis showed that human U6 promoter was amplified from genomic DNA (Figure 1A). Restriction digestion analysis and DNA sequencing indicated a vector suitable for siRNAs expression in mammalian cells, pUC18U6, was successfully constructed (Figure 1B and Figure 2A). We then cloned into pUC18U6, downstream of U6 promoter, the annealed two oligodeoxyribonucleotides encoded a short-hairpin RNA (shRNA) against HBV. shRNAs could be processed into siRNA in cells[24,25]. Since there was no Xho I site in pUC18U6, we designed the spacer (CTCGAG) in shRNA recognized by Xho I. Therefore, the recombinant vector pUC18U6HBVsir containing the shRNA coding sequence could yield a new 310 bp fragment compared with pUC18U6 after digested with Xho I and Hind III (Figure 2B), and this was verified by agarose gel analysis (Figure 1C). This indicated that pUC18U6HBVsir which could express a siRNA against HBV, was successfully constructed (Figure 2B).
Figure 1 Construction of mammalian siRNA expression vector pUC18U6 and HBV-siRNA expression vector pUC18U6HBVsir.
A: Amplification of human U6 promoter by PCR. 1: DNA marker (2000, 1000, 750, 500, 250, 100 bp from top to bottom); 2: PCR product. B: Restriction digestion analysis of recombinant vector pUC18U6. 1: pUC18; 2: pUC18U6; 3: DNA marker (2000, 1000, 750, 500, 250, 100 bp from top to bottom). C: Restriction digestion analysis of recombinant vector pUC18U6HBVsir. 1: pUC18U6HBVsir; 2: pUC18; 3: DNA marker (2000, 1000, 750, 500, 250, 100 bp from top to bottom).
Figure 2 Maps of pUC18U6 and pUC18U6HBVsir.
A: pUC18U6. B: pUC18U6HBVsir.
Effect of pUC18U6HBVsir expressed siRNA on HBV replication
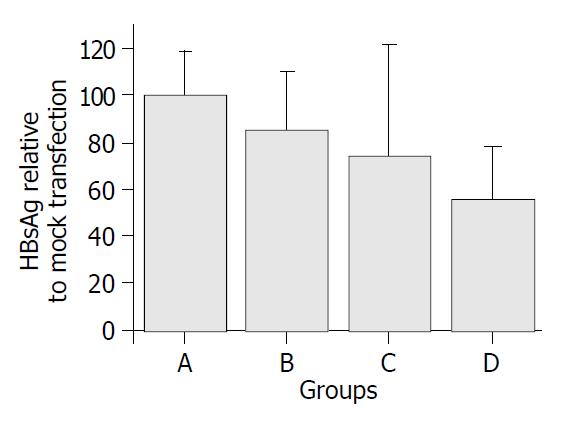
pUC18U6HBVsir and its control plasmids were transfected into 2.2.15 cells by using liposome. Forty-eight hours after the transfection, the supernatant of the cell culture was collected, and HBsAg in the supernatant was quantified as described in Materials and Methods. As shown in Figure 3, transfection of pUC18U6HBVsir reduced HBsAg significantly as compared with the controls (P < 0.05), suggesting that siRNA expressed by pUC18U6HBVsir suppressed the replication of HBV, and this suppression was specific because transfection of pUC18U6GFPsir, which expressed siRNA against GFP, had no effect on HBV replication.
Figure 3 HBsAg concentrations in supernatants of transfected 2.
2.15 cells. Groups A-D represent mock-transfected 2.2.15 cells or 2.2.15 cells transfected by pUC18U6, pUC18U6GFPsir and pUC18U6HBVsir, respectively. The concentration of HBsAg in the supernatant of 2.2.15 cells transfected by pUC18U6HBVsir was decreased by 44% as compared with that of mock-trans-fected 2.2.15 cells.
DISCUSSION
The aim of the present study was to explore siRNAs as a novel strategy in the treatment of HBV infection. Firstly, we first constructed a vector which was suitable to express siRNAs in mammalian cells. siRNAs could be produced in vitro and in vivo. In vitro, siRNAs could be produced by chemical synthesis, in vitro transcription, or by enzymatic digestion of dsRNAs[26-29]. siRNAs produced in vitro could be transfected directly into mammalian cells to knockdown gene expression. However, in vitro produced siRNAs have several shortcomings, such as transient effect, and high cost. To overcome these shortcomings, several groups have developed a couple of DNA-vectors which could express siRNAs in vivo[11,23-25,27,28]. These vectors had different promoters and different backbones, and expressed siRNAs via two approaches: sense and antisense strands of siRNAs transcribed from tandem promoters and then combined to form siRNAs, or short hairpin RNAs transcribed from a single promoter and then processed by Dicer, a key enzyme in RNA interference, to yield siRNAs. The mammalian siRNAs expression vector constructed by us used human U6 promoter, a pol III promoter, to drive the expression of shRNAs. Compared with other reported vectors, our vector has some features which make the cloning of shRNA expression cassettes easy. The Kpn I and EcoR I sites downstream of the U6 promoter were used for directional cloning of shRNA expression cassettes and the first nucleotide of the Kpn I site was guanosine, just the required nucleotide for the +1 position of the U6 promoter. Therefore, there was no need to consider this requirement when we designed shRNA sequences by using our vector. Additionally, our vector has no Xho I site. By incorporating Xho I site as the spacer in the shRNA sequences, it is easy to identify positive shRNA expression vectors containing shRNA expression cassettes by routine agarose gel analysis of Xho I/Hind III digestion products of the vectors. Positive vectors should reveal a new small fragment of about 300 bp which was absent from the negatives (Figure 1C and Figure 2B). Some reported vectors also identify positive shRNA expression by double enzyme digestion. However, the double enzyme digestion sites in these vectors are just the sites used for cloning of shRNA expression cassettes. Therefore, for these vectors it is not easy to identify the positive shRNA expression vectors by routine agarose gel analysis, especially minigel analysis widely used in laboratories, since shRNA expression cassettes are generally 50-60 bp in length, almost the limit of resolving power of routine agarose gel analysis. The same constraint of routine agarose gel analysis also requires shRNA expression cassettes without double enzyme digestion sites, and limit siRNA target site selection. In contrast, there was no such limitation when using our vector to express siRNAs.
Several groups have reported using siRNAs to inhibit HBV replication in cell cultures and animal models[12-19]. Although all of these reports showed that HBV replication was inhibited to various degrees by siRNAs, several factors must be taken into account before siRNAs are used to treat HBV infection. One is target selection. siRNAs target at different sites of the same gene can vary from strong to less inhibition of the gene expression. Up to now, little is known about the mechanism of this selection. Therefore, it is still an empirical matter to design the most effective siRNAs. In our study, we chose 1086-1103 nt of HBV genome as the target, different from other groups. Our results suggested that siRNA targeting this site specifically inhibited HBV replication. Considering that HBV genome is 3.2 kb and there are no general rules to predetermine the most effective siRNAs, a systematic comparison by experiments of the efficiency of siRNAs against different targets of HBV genome in inhibiting HBV replication should be done to pick out the most effective siRNAs for treating HBV infection. Another concern of using siRNAs for treatment of HBV infection is viral mutants. RNAi is so specific in gene silencing that even a single-base mismatch between siRNAs and cognate genes can significantly reduce the silencing effect. Since viruses evolve very rapidly, they can gain the resistance to once-effective siRNA molecules in short time. In fact, two recent studies reported HIV and poliovirus escape mutants after extended siRNAs treatment[30,31]. To overcome this obstacle, multiple viral sequences must be targeted simultaneously. In this aspect, our study adds another target when designing multi-target siRNAs to inhibit HBV replication. Further studies are needed to determine the optimal combination of these targets.