Published online Jul 1, 2004. doi: 10.3748/wjg.v10.i13.1872
Revised: January 1, 2004
Accepted: January 8, 2004
Published online: July 1, 2004
AIM: To obtain human recombinant Fv-immunotoxin hscFv25-mTNFα (mutant human TNFα fused to human scFv25) against hepatocellular carcinoma (HCC).
METHODS: Two relevant sites of enzymatic digestion were added to mTNFα by PCR. mTNFα was linked to the 3’ end of hscFv25 in pGEX4T-1 vector. This anti-HCC recombinant Fv-immunotoxin hscFv25-mTNFα was expressed in Escherichia coli and purified from inclusions. After purified by glutathione-S-transferase affinity chromatography and thrombin digestion, it was identified by electrophoresis and Western blot. And then, the purified recombinant Fv-immunotoxin was injected into nude mice with HCC xenografts through their tail veins. mTNFα protein and PBS were used as control at the same time. After treated for two weeks, nude mice were executed. The bulk and weight of tumors were observed. The tumor tissues were stained by immunohistochemical method with TNFα antibody.
RESULTS: The expression ratio of recombinant Fv-immunotoxin hscFv25-mTNFα was 12% of bacterial protein. The result of tumor restraining trials of hscFv25-mTNFα showed 2/5 complete remission and 3/5 partial remission. mTNFα restraining trials showed 5/5 partial remission. The therapeutic result of hscFv25-mTNFα was better than that of mTNFα (F = 8.70, P < 0.05). The hscFv25-mTNFα remedial tumor tissues were positive for TNFα by immunohistochemical staining. The positive granules mainly existed in the cytoplasm of tumor cell.
CONCLUSION: Recombinant Fv-immunotoxin hscFv25-mTNFα has better therapeutic effect than mTNFα. It can inhibit the cellular growth of HCC and has some potential of clinical application.
- Citation: Zhang J, Liu YF, Yang SJ, Qiao Q, Cheng H, Zhang CS, Ma FC, Guo HZ. Primary targeting of recombinant Fv-immunotoxin hscFv25-mTNFα against hepatocellular carcinoma. World J Gastroenterol 2004; 10(13): 1872-1875
- URL: https://www.wjgnet.com/1007-9327/full/v10/i13/1872.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i13.1872
Hepatocellular carcinoma (HCC) is a common malignant tumor in China with poor prognosis[1,2]. Its diagnosis and therapy are still major challenges[3,4]. The single chain Fv (scFv) is consisted of heavy-chain and light-chain variable region of the antibody, linked with a peptide chain. They have shown a various application value in tumor therapy[5-7]. Our laboratory, through collaboration with the Academy of Military Medical Science, constructed human scFv25 against HCC (hscFv25) several years ago[8]. In this study, we fused human mutant tumor necrosis factor-alpha (mTNFα) to hscFv25 and constructed prokaryotic expressing vector pGEX4T-1/hscFv25-mTNFα. After recombinant Fv-immunotoxin hscFv25-mTNFα was expressed, purified and identified, we used it in tumor restraining trials of the HCC (SMMC-7721) xenografts in nude mice.
Isopropyl-1-thio-D-galactopyranoside (IPTG), PCR marker, T4 ligase and thrombin were purchased from Promega. Glutathione-S-transferase affinity chromatogram and low molecular weight marker were purchased from Pharmacia. The plasmid extracting reagent kit was purchased from Huashun Biologic Co. (Shanghai, China). EnVisionTM System was purchased from Dako. The mTNFα protein produce was offered by Biologic Center of the Fourth Military Medical University. The mouse anti-human TNFα monoclonal antibody was purchased from Santa Cruz, USA.
According to cDNA of mTNFα, Sal I site was added to its 5’-end and Xhol I site to its 3’-end. The 5’ primer was: ACTCTCGAGTCAGAAGGCAATGATCCCAAAGTA; and the 3’ primer was: ACGCGTCGACCGCAAACGTAAGCCTGTA.
The primers were synthesized by Sangon (Shanghai, China). After digested by Sal I and Xhol I restriction enzymes, mTNFα PCR products were linked to 3’-end of pGEX4T-1/hscFv25, which was digested by the same restriction enzymes. And then, pGEX4T-1/hscFv25-mTNFα was transformed into E. coli. JM109. E. coli. were cultivated in the Amp+/LB medium at 37 °C for 16-22 h. The positive clones were selected and identified by EcoR I and Sal I, EcoR I and Xhol I, Sal I and Xhol I restriction enzymes analysis and 10 g/L agarose gel electrophoresis.
E. coli. were cultivated at 37 °C in LB medium containing 50 g/L ampicillin. When their density reached A650 = 1.0, bacteria were induced by 1.0 mmol/L IPTG for 3-4 h. And then they were harvested by centrifugation and their inclusions were isolated, purified, denatured and re-natured. After purified by glutathione-S-transferase affinity chromatography, glutathione-S-transferase (GST) was removed by thrombin digestion. The immunotoxin was analyzed by 120 g/L SDS-PAGE.
When 120 g/L SDS-PAGE electrophoresis was completed, stacking gel was removed and equilibrated in transfer buffer (25 mmol/L Tris, 190 mmol/L glycocine, 200 mL/L methanol). The expressing proteins were transferred from gel to NC membrane by electrophoresis. The membrane in heat-sealable plastic bag was blocked with buffer (TBS with 30 g/L bovine serum albumin, BSA) overnight at 4 °C. After the blocking buffer was removed, the solution with TNFα antibody was added, which was diluted with TBS (containing 10 g/L BSA). And then the membrane was incubated for 1h at 37 °C. After washed 3 times with TBS, the membrane was transferred to fresh plastic bag containing goat anti-mouse IgG antibody for one hour at 37 °C and stained with DAB for 10-20 min.
The SMMC-7721 cells were cultivated in RPMI 1640 containing 100 mL/L fetal bovine serum, which was obtained from Gibco BRL. Fifteen immunodeficient nude mice BALB/nu, purchased from our Experiment Animal Center and Shanghai Cellular and Biologic Center, were implanted s.c. 2 × 106 SMMC-7721 cells (PBS suspended) at right rear flank respectively. When tumors grew to a palpable size about 2 mm subcutaneously, fifteen mice were divided into three groups randomly. Each group had five mice. PBS was injected to the first group of mice. Twelve μg mTNFα was injected to each mouse in the second group and 16 μg hscFv25-mTNFα was injected to each mouse in the third group. All mice were injected through their tail veins. Fourteen days were one course of therapy. At the end of the second week, the mice were dissected. Their tumors were weighed and examined for morphological evidence of damage, along with lungs and livers. The paraffin sections were prepared for histological examination and immunohistochemical staining.
The sections were stained by immunohistochemical method. Those in the control groups were stained according to the same method, with the first antibody substituted by PBS, normal mouse serum and an irrelevant antibody IB3 respectively. All paraffin embedded samples were deparaffined and rehydrated, pretreated for 20 min at 95 °C in a microwave oven. After being treated with 3 mL/L H2O2 for 30 min to block the endogenous peroxidase, the sections were incubated with 20 mL/L fetal calf serum for 30 min to reduce nonspecific binding, and then the primary TNFα antibody was applied to sections at 4 °C overnight. The sections were subsequently incubated with horseradish peroxidase (HR)-labeled goat anti-mouse immunoglobulin at 37 °C for 1 h, and stained with DAB-H2O2 for 5-10 min and counterstained with hematoxylin.
The F test was used for statistical analysis of tumor bulk and weight among three groups. The criterion of significance was set at P < 0.05.
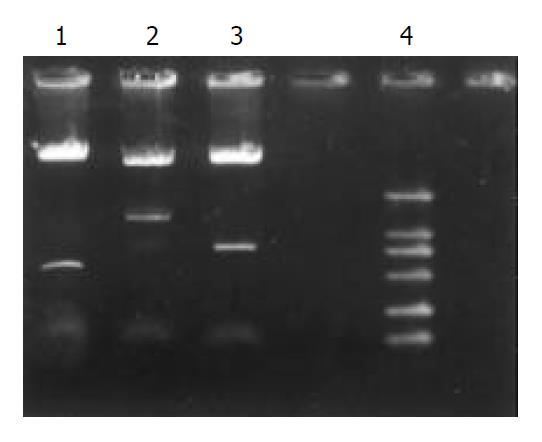
One ladder was observed at 450 bp, with the same size as mTNFα. After PCR products were digested by Sal I, Xhol I and linked to 3’-end of pGEX4T-1/hscFv25, which was digested by the same restriction enzymes, the prokaryotic expressing vector pGEX4T-1/hscFv25-mTNFα was constructed. Then it was transformed to E.coli JM109. The bacteria were cultivated in the Amp+/LB medium at 37 °C for 16-22 h. Six clones were selected at random. Three clones had one ladder about 450 bp digested by Sal I and Xhol I restriction enzymes. After subsequently digested by EcoR I and Xhol I, EcoR I and Sal I, 1180 bp and 730 bp ladders were observed, corresponding well with the size of hscFv25-mTNFα and hscFv25 (Figure 1).
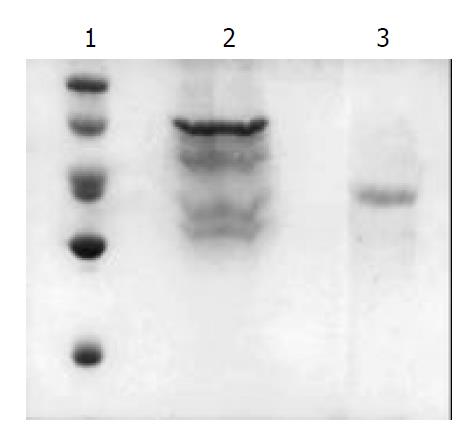
The bacteria containing pGEX4T-1/hscFv25-mTNFα were induced by IPTG. Compared with the same bacteria without induction by IPTG, there was a new transcript of Mr 68000, corresponding well with the size of fusion protein GST– hscFv25-mTNFα (GST Mr 26000, hscFv25Mr 26000, mTNFαMr 16000). The result of absorbance scanning showed that the expressing amount of GST-hscFv25-mTNFα was 12% bacterial protein. The fusion protein existed in the form of infusibility inclusions. After the inclusions were denatured, renatured, purified by affinity chromatography, only 2 mg preliminarily purified GST–hscFv25-mTNFα was obtained from 100 mL bacteria. Digested by thrombin subsequently and purified by GST affinity chromatography again, hscFv25-mTNFα protein was obtained. The result of SDS-PAGE showed that proteins were accorded to electrophoresis purification (Figure 2). The GST-hscFv25-mTNFα studied by Western blot was seen as a new transcript of Mr 68000, the same as the fusion protein GST-hscFv25-mTNFα.
Fifteen nude mice, weighing 16-24 g, were implanted s.c. 2 × 106 SMMC-7721 cells at their right rear flanks and received drug treatment on the tenth day. The tumor of bulk and weight of 15 nude mice were shown in Table 1. The tumor restraining trials of hscFv25-mTNFα to HCC xenografts showed 2/5 complete remission (CR) and 3/5 partial remission (PR). The therapeutic result of mTNFα showed 5/5 PR. The therapeutic effectiveness of mTNFα was less than that of hscFv25-mTNF-α (F = 8.70, P < 0.05). In the PBS group, tumors showed no remission (NR). After the liver and lung specimens were sliced up continuously and examined, no metastastic tumors and surviving tumor cells were seen in CR xenografts. The tissues of PR xenografts showed a large number of necrotic areas.
| Tumor bulk | Tumor weight | Regression rate of | Regression rate of | Curative | ||
| (mm × mm) | (mg) | tumor bulk (%) | tumor weight (%) | effect | ||
| PBS | 1 | 9 × 5 | 119 | 0 | 0 | NR |
| 2 | 7 × 7 | 87 | 0 | 0 | NR | |
| 3 | 9 × 4 | 51 | 0 | 0 | NR | |
| 4 | 5 × 3 | 26 | 0 | 0 | NR | |
| 5 | 5 × 4 | 30 | 0 | 0 | NR | |
| hscFv25 | 1 | 0.8 × 0.5 | 2 | 99.2 | 96.8 | PR |
| -mTNFα | 2 | 2 × 3 | 10 | 81.8 | 84.0 | PR |
| 3 | 2 × 3 | 15 | 81.8 | 76.0 | PR | |
| 4 | 0 | 0 | 100 | 100 | CR | |
| 5 | 0 | 0 | 100 | 100 | CR | |
| mTNFα | 1 | 2 × 2 | 11 | 87.9 | 82.4 | PR |
| 2 | 3 × 3 | 22 | 72.7 | 64.9 | PR | |
| 3 | 2 × 4 | 5 | 75.8 | 76.0 | PR | |
| 4 | 2 × 3 | 10 | 81.8 | 84.0 | PR | |
| 5 | 2 × 3 | 10 | 81.8 | 84.0 | PR | |
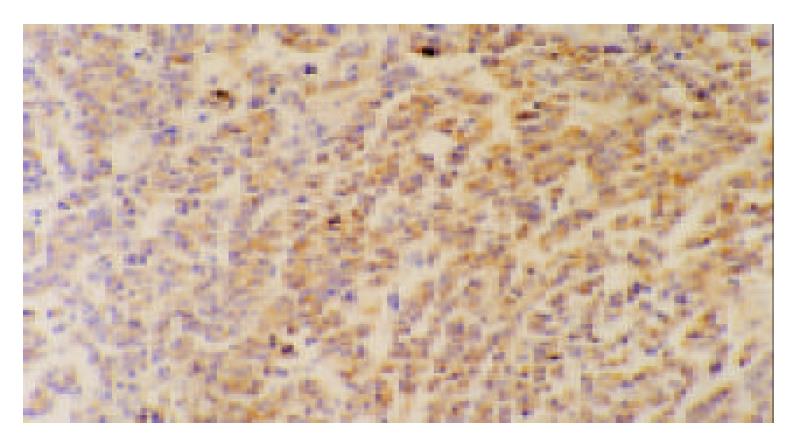
The remnant tumor tissues treated by hscFv25-mTNFα showed diffusely positive for TNFα. The positive granules mainly existed in the cytoplasm of tumor cells (Figure 3). Immunohistochemical staining with the irrelevant antibody IB3, PBS and normal mouse serum was negative. The tumor tissues treated by PBS showed negative or weak positive for TNFα.
In the past 20 years, the antibody study has passed through 3 stages: polyclonal antibody, monoclonal antibody and genetic engineering antibody. Among them, the genetic engineering antibody was the most arresting focus of research because of its prominent advantage, such as lower molecular weight, better specificity and affinity[9-12]. It had minimal antigenicity of human against mouse immunoglobulin antibody (HAMA)[13]. Many scFvs against tumors have been used in the phase I-III clinical therapy trials[14-16]. HAb25 is a kind of the monoclonal antibody against HCC constructed by our laboratory. The hscFv25 against HCC is constructed from HAb25. Previous experiment showed that it had better targeting action compared to HAb25[17-19].
The recombinant Fv-immunotoxin was constructed if the scFv C’end was linked to protein toxins, such as pseudomonas exotoxin (PE)[20]. Once bound to the target cells, immunotoxins could kill the tumor cells. Due to PE being nonhuman, to produce effect, it must be internalized into endocytic vesicles where the catalytic protein of the toxin was processed and released into cytosol. Some results of clinical trials using such exotoxin were not satisfactory. Here, we have constructed the single chain recombinant Fv-immunotoxin hscFv25-mTNFα. The mTNFα we used was a mutant from natural human TNFα.
The TNFα is one of the strongest active factors in organisms which can kill tumor cells directly. It has severe side effects and is often used in local therapy or targeting therapy by antitumor antibodies[21,22]. Some studies found that the toxicity of TNFα could be reduced by genic fix point mutation or polymeric alteration[23,24]. When natural TNFα’s N-end seven amino acids were cut out and Pro8Ser9Asp10 changed to ArgLysArg, and its C-end 157th amino acid of Leu changed to Phe, mTNFα was attained. Compared with natural TNFα, the toxicity of mTNFα was reduced evidently and its cytotoxicity was enhanced greatly[25].
Our study showed the hscFv25-mTNFα could kill tumor cells effectively. Compared with dosage of mTNFα and the former experimentation[17], the dosage of hscFv25-mTNFα was reduced markedly. Those results revealed that mTNFα could concentrate in tumor tissues and killed tumor cells by the targeting of hscFv25. The anti-HCC recombinant Fv-immunotoxin with TNFα replaced by mTNFα could increase its cytotoxicity. Furthermore, we found tumor cells were positive for TNFα in immunohistochemical staining. It also indicated hscFv25 had better targeting action.
All these showed the hscFv25-mTNFα had better distributive ratio in HCC tissues. It could help local tumor cells to attain a high level of mTNF-α and reduce dose-limiting toxicity. The recombinant Fv-immunotoxin hscFv25-mTNFα might have potentiality of clinical application. Due to few nude mice in our study, it maybe lacked rigorous statistical signification. The experimental results need be approved in the future.
Edited by Zhu LH Proofread by Xu FM
| 1. | Qian J, Feng GS, Vogl T. Combined interventional therapies of hepatocellular carcinoma. World J Gastroenterol. 2003;9:1885-1891. [PubMed] |
| 2. | Zeng ZX, Wang WL, Luo WJ, Wang ZL. Construction of differ-entially expressed cDNA library in human hepatocellular carci-noma apoptotic cells with suppression subtractive hybridization. Shijie Huaren Xiaohua Zazhi. 2001;9:1233-1237. |
| 3. | Qi YY, Zhou LG, Wang WX, Chen WJ, Xiang KL, Zhao XY, Yang TH, Yi XZ. Duai-phase enhanced spiral CT in the diagnosis of hepatocellular carcinoma with tumor thrombus in portal vein. Shijie Huaren Xiaohua Zazhi. 2002;10:384-387. |
| 4. | Zhen JY, Li KZ, Wang WZ. Impact of the expression of P27KIPI on apoptosis and progression of hepatocellular carcinoma. Shijie Huaren Xiaohua Zazhi. 2002;10:883-886. |
| 5. | Rosenblum MG, Cheung LH, Liu Y, Marks JW. Design, expression, purification, and characterization, in vitro and in vivo, of an antimelanoma single-chain Fv antibody fused to the toxin gelonin. Cancer Res. 2003;63:3995-4002. [PubMed] |
| 6. | Reinartz S, Hombach A, Köhler S, Schlebusch H, Wallwiener D, Abken H, Wagner U. Interleukin-6 fused to an anti-idiotype antibody in a vaccine increases the specific humoral immune response against CA125+ (MUC-16) ovarian cancer. Cancer Res. 2003;63:3234-3240. [PubMed] |
| 7. | Goel A, Colcher D, Baranowska-Kortylewicz J, Augustine S, Booth BJ, Pavlinkova G, Batra SK. Genetically engineered tetravalent single-chain Fv of the pancarcinoma monoclonal antibody CC49: improved biodistribution and potential for therapeutic application. Cancer Res. 2000;60:6964-6971. [PubMed] |
| 8. | Yuan QA, Yu WY, Huang CF. [Construction and expression of a hepatocellular carcinoma specific rodent and its humanized single-chain Fv fragments in Escherichia coli]. Shengwu Gongcheng Xuebao. 2000;16:86-90. [PubMed] |
| 9. | Niv R, Assaraf YG, Segal D, Pirak E, Reiter Y. Targeting multidrug resistant tumor cells with a recombinant single-chain FV fragment directed to P-glycoprotein. Int J Cancer. 2001;94:864-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | McCall AM, Shahied L, Amoroso AR, Horak EM, Simmons HH, Nielson U, Adams GP, Schier R, Marks JD, Weiner LM. Increasing the affinity for tumor antigen enhances bispecific antibody cytotoxicity. J Immunol. 2001;166:6112-6117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Cooke SP, Boxer GM, Lawrence L, Pedley RB, Spencer DI, Begent RH, Chester KA. A strategy for antitumor vascular therapy by targeting the vascular endothelial growth factor: receptor complex. Cancer Res. 2001;61:3653-3659. [PubMed] |
| 12. | van der Poel HG, Molenaar B, van Beusechem VW, Haisma HJ, Rodriguez R, Curiel DT, Gerritsen WR. Epidermal growth factor receptor targeting of replication competent adenovirus enhances cytotoxicity in bladder cancer. J Urol. 2002;168:266-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Whittington HA, Hancock J, Kemshead JT. Generation of a humanised single chain Fv (Scfv) derived from the monoclonal Eric-1 recognising the human neural cell adhesion molecule. Med Pediatr Oncol. 2001;36:243-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Turatti F, Mezzanzanica D, Nardini E, Luison E, Maffioli L, Bambardieri E, de Lalla C, Canevari S, Figini M. Production and validation of the pharmacokinetics of a single-chain Fv fragment of the MGR6 antibody for targeting of tumors expressing HER-2. Cancer Immunol Immunother. 2001;49:679-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Mayer A, Tsiompanou E, O'Malley D, Boxer GM, Bhatia J, Flynn AA, Chester KA, Davidson BR, Lewis AA, Winslet MC. Radioimmunoguided surgery in colorectal cancer using a genetically engineered anti-CEA single-chain Fv antibody. Clin Cancer Res. 2000;6:1711-1719. [PubMed] |
| 16. | Chester KA, Bhatia J, Boxer G, Cooke SP, Flynn AA, Huhalov A, Mayer A, Pedley RB, Robson L, Sharma SK. Clinical applications of phage-derived sFvs and sFv fusion proteins. Dis Markers. 2000;16:53-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Sun Z, Liu Y, Yuan Q, Yu W, Huang C, Ma L, Yu J. [Targeting studies of humanized scFv25 fusing to TNFalpha against hepatocellular carcinoma]. Zhonghua Ganzangbing Zazhi. 2000;8:352-354. [PubMed] |
| 18. | Cheng H, Liu YF, Zhang HZ, Shen WN, Zhang J, Zhang J. In vivo antitumour activity of PBMCs via genetic modification of single-chain immunotoxin. Shijie Huaren Xiaohua Zazhi. 2003;11:708-711. |
| 19. | Cheng H, Liu YF, Zhang HZ, Shen WN, Zhang J, Zhang J. In vitro cytotoxicity of PBMCs via genetic modification of single-chain immunotoxin. Shijie Huaren Xiaohua Zazhi. 2003;11:281-284. |
| 20. | Fan D, Yano S, Shinohara H, Solorzano C, Van Arsdall M, Bucana CD, Pathak S, Kruzel E, Herbst RS, Onn A. Targeted therapy against human lung cancer in nude mice by high-affinity recombinant antimesothelin single-chain Fv immunotoxin. Mol Cancer Ther. 2002;1:595-600. [PubMed] |
| 21. | Thomas PS, Heywood G. Effects of inhaled tumour necrosis factor alpha in subjects with mild asthma. Thorax. 2002;57:774-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Cooke SP, Pedley RB, Boden R, Begent RH, Chester KA. In vivo tumor delivery of a recombinant single chain Fv: : tumor necrosis factor-alpha fusion [correction of factor: a fusion] protein. Bioconjug Chem. 2002;13:7-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Yamamoto M, Oshiro S, Tsugu H, Hirakawa K, Ikeda K, Soma G, Fukushima T. Treatment of recurrent malignant supratentorial astrocytomas with carboplatin and etoposide combined with recombinant mutant human tumor necrosis factor-alpha. Anticancer Res. 2002;22:2447-2453. [PubMed] |
| 24. | Terlikowski SJ. Local immunotherapy with rhTNF-alpha mutein induces strong antitumor activity without overt toxicity--a review. Toxicology. 2002;174:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Nakamura S, Kato A, Masegi T, Fukuoka M, Kitai K, Ogawa H, Ichikawa Y, Maeda M, Watanabe N, Kohgo Y. A novel recombinant tumor necrosis factor-alpha mutant with increased anti-tumor activity and lower toxicity. Int J Cancer. 1991;48:744-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |