Published online Jul 1, 2004. doi: 10.3748/wjg.v10.i13.1867
Revised: February 14, 2004
Accepted: February 21, 2004
Published online: July 1, 2004
AIM: To investigate the expression of adenovirus-mediated human endostatin (Ad/hEndo) gene transfer and its effect on the growth of hepatocellular carcinoma (HCC) BEL-7402 xenografted tumors.
METHODS: Immunohistochemistry analysis with an anti-endostatin antibody was preformed to detect endostatin protein expression in HCC BEL-7402 cells infected with Ad/hEndo. MTT assay was used to investigate the effects of Ad/hEndo on proliferation of human umbilical vein endothelial cells (HUVEC). Intra-tumoral injections of 1 × 109 pfu Ad/hEndo was given to treat BEL-7402 xenografted tumors in nude mice once weekly for 6 wk. Mice received injections of Ad/LacZ and DMEM were regarded as control groups. After intra-turmoral administration with Ad/hEndo, the endostatin mRNA expression in tumor tissue was analyzed by Northern blotting, and plasma endostatin levels were determined using enzyme-linked immunosorbent assay (ELISA).
RESULTS: High level expression of endostatin gene was detected in the infected HCC BEL-7402 cells. Ad/hEndo significantly inhibited HUVEC cell proliferation by 57.2% at a multiplicity of infection (MOI) of 20. After 6-week treatment with Ad/hEndo, the growth of treated tumors was inhibited by 46.50% compared to the Ad/LacZ control group (t = 2.729, P < 0.05) and by 48.56% compared to the DMEM control group (t = 2.485, P < 0.05). The ratio of mean tumor volume in treated animals to mean tumor volume in the control animals (T:C ratio) was less than 50% after 24 d of treatment. Endostatin mRNA in tumor tissue was clearly demonstrated as a band of approximately 1.2 kb, which was the expected size of intact and functional endostatin. Plasma endostatin levels peaked at 87.52 ± 8.34 ng/mL at d 3 after Ad/hEndo injection, which was significantly higher than the basal level (12.23 ± 2.54 ng/mL). By d 7, plasma levels dropped to nearly half the peak level (40.34 ± 4.80 ng/mL).
CONCLUSION: Adenovirus-mediated human endostatin gene can successfully express endogenous endostatin in vitro and in vivo, and significantly inhibit the growth of BEL-7402 xenografted liver tumors in nude mice.
- Citation: Li L, Huang JL, Liu QC, Wu PH, Liu RY, Zeng YX, Huang WL. Endostatin gene therapy for liver cancer by a recombinant adenovirus delivery. World J Gastroenterol 2004; 10(13): 1867-1871
- URL: https://www.wjgnet.com/1007-9327/full/v10/i13/1867.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i13.1867
Increasing evidence suggests that solid tumors and their metastasis are angiogenesis dependent[1]. Angiogenesis, the formation of new microvessels generated by vascular endothelial cells, provides essential nutrition for rapid growth of malignant cells. Without angiogenesis, neoplasm might remain in dormancy[2]. Anti-angiogenesis therapies, which block the blood supply of a growing tumor by inhibiting proliferation of endothelial cells, provide a new strategy for treatment of solid tumors[3]. Vascular endothelial cells, the target of anti-angiogenic treatment are genetically stable. So it is unlikely that anti-angiogenic agents would induce the drug resistance[4].
Transcatheter arterial embolization (TAE) has been widely practiced in the treatment of unresectable hepatocellular carcinoma (HCC)[5]. However, the obstruction of hepatic artery induces extensive ischemic necrosis or hypoxia which are strongly correlated with increased expression of angiogenic factors, such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF)[6]. The proliferative activity of vascular endothelial cells and tumour cells was increased in residual tumor following arterial embolization[7]. Combination of antiangiogenesis and TAE therapy could inhibit increased proliferation of vascular endothelial cells and capillary vessel formation induced by arterial embolization, and improve the therapeutic effect[5].
Endostatin, a carboxyl-terminal proteolytic fragment of collagen X VIII, was regarded as one of the most potent inhibitors of tumor angiogenesis[8]. Several studies have shown that recombinant endostatin protein generated from Escherichia coli and Pichia pastoris yeast significantly inhibited growth and metastasis of xenografted tumor in different tumor models with no drug resistance and side effects[9,10]. However, its widespread application has been hampered by difficulties in the large-scale production of high bioactive protein[11,12].
In this study, we constructed a replication-defective recombinant adenoviral vector harboring a human endostatin gene (Ad/hEndo), and demonstrated efficient expression of endostatin gene in vivo and its antitumoral effect on BEL-7402 xenografted liver tumors in nude mice.
Cells and adenoviruses BEL-7402 cells (Hepatocellular carcinoma, HCC) and human umbilical vein endothelial cells (HUVEC) were maintained in RPMI 1640 supplemented with 100 mL/L fetal bovine serum (FBS). The culture medium contained 100 U/mL of penicillin and 100 µg/mL of streptomycin (GIBCO BRL, Gathersburg, MD).
Recombinant adenovirus vectors carrying the human endostatin gene (Ad/hEndo) and LacZ gene (Ad/LacZ) were generated as described previously[12,13]. All virus particles were amplified in 293 cells and purified by cesium chloride gradient centrifugation. Viruses were titered using a standard plaque-forming unit (pfu) assay.
Animals Four to 6-week-old male BALB/c nude mice (with body weight of 19 to 23 g) were purchased from Animal Experiment Center (Medial College, Sun Yat-sen University) and maintained in a specific pathogen-free (SPF) environment (certificate: 26-99S029).
Agents Human endostatin protein accucyte EIA (Oncogene Research Products, Boston, MA), rabbit anti-human endostatin polyclonal antibody (Chemicon International, Temecula, CA).
Immunohistochemistry for expression of endostatin protein in vitro BEL-7402 cells were infected with Ad/hEndo at a multiplicity of infection (MOI) of 10. At 2 h postinfection, the medium was replaced with Dulbecco’s minimal essential medium (DMEM) containing 100 mL/L FBS. After 72-h incubation, cells were collected and plated on slides. The slides were washed, fixed and blocked for 20 min in Tris-buffered saline (TBS) containing 10 mL/L goat serum, then incubated with rabbit anti-human endostatin polyclonal antibody diluted 1:50 in PBS overnight. After washing 3 times, biotinylated goat anti-rabbit secondary antibody (Dako Corp., Carpinteria, CA) diluted 1:200 in PBS was added, and slides were incubated for an additional 30 min. Slides were then washed 3 times with PBS and stained with streptavidin-biotinylated horseradish peroxide complex.
HUVEC cells proliferation assay Human umbilical vein endothelial cells (HUVEC) were seeded in a 96-well plate at a density of 1 × 104/well and allowed to adhere overnight. Cells were then infected for 2 h with Ad/hEndo at MOIs of 0.05, 0.5, 1.0, 5.0, 10 and 20, and with Ad/LacZ at an MOI of 20. Cells were washed 3 times with PBS and incubated at 37 °C in fresh medium. Seventy-two hours later, 20 µL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) (Fluka Biochemika, Buchs, Switzerland) was added to the wells and the plate was incubated for an additional 4 h at 37 °C. Then, 100 µL DMSO was added to the wells and the plate was incubated for 10 min at 37 °C. Absorbance was read using a Bio-Rad 550 microplate reader (Bio-Rad Laboratories, Hercules, CA) at 570 nm. The value of the treated cells was calculated as percent of the untreated control.
In vivo tumor growth inhibition BEL-7402 cells were subcutaneously inoculated into the flanks of 6-week-old female BALB/c nude mice (5 × 106 cells in 200 µL). Ten days after tumor cell inoculation, intra-tumoral injections of virus (Ad/hEndo or Ad/LacZ; 1 × 109 pfu in 100 µL) was given once weekly for 6 wk. An additional 7 animals received injections of 100 µL of DMEM as a negative control. Tumors were measured with a caliper gauge twice a week over a 6-week period following the initial virus injections. Tumor volume was calculated according to the formula: volume = width2× length × 0.52[10]. One week after the 6th injection of Ad/hEndo, the mice were sacrificed and the xenografted tumors were excised and weighed. The inhibition rates of growth of BEL-7402 xenografted tumors were calculated according to the formula: Inhibition rate (%) = (1-mean Weight(Ad/hEndo)/mean Weight(control)) × 100.
Northern blotting for expression of endostatin mRNA in vivo On d 1, 4 and 8 after intra-tumoral administration with 100 µL of 1 × 109 pfu Ad/hEndo, the mice were sacrificed. The xenografted tumors were excised and snap frozen in liquid nitrogen. Total RNA was isolated from tumor tissues using the Trizol reagent (Invitrogen) according to the manufacturer’s instructions. RNA isolated from the tumors injected with DMEM was used as a negative control. Total RNA of 15 µg was resolved on a 10 g/L formaldehyde agarose gel, and transferred to nylon membrane. The RNA was fixed onto the membrane by UV cross-linking. The membrane was prehybridized in DIG Easy Hyb hybrization solution (Roche, Basel, Switzerland) at 68 °C for 30 min, then hybridized in DIG Easy Hyb hybrization solution containing digoxin (DIG)-labeled endostatin probe at 68 °C overnight. The membrane was incubated with anti-DIG antibody for 30 min and stained with NBT/BCIP for 10 min.
Plasma endostatin levels detected by ELISA Blood samples were harvested on d 0, 3 and 7 after intra-tumoral administration with 100 µL of 1 × 109 pfu Ad/hEndo. Samples were centrifuged, and plasma endostatin levels were determined using a human endostatin enzyme-linked immunosorbent assay (ELISA) kit (Oncogene Research Products, Boston, MA), according to the manufacturer’s instructions.
Pathological observation One week after the 6th course of treatment, the animals were sacrificed. The major organs including heart, liver, spleen, kidney, lung and brain were extracted from the nude mice, fixed in 40 g/L buffered formaldehyde phosphate, cut into 4 µm thick for tissue section and stained with haematoxylin and eosin.
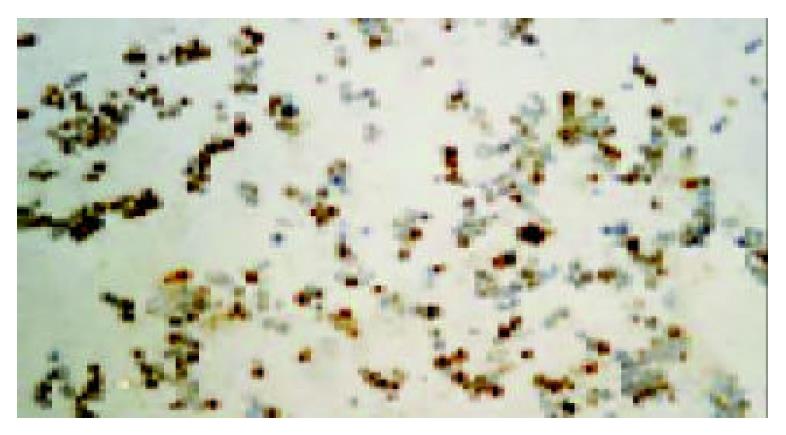
Three days after transfection with Ad/hEndo at a MOI of 10, BEL-7402 cells were probed with rabbit anti-human endostatin antibody. Immunohistochemistry with an anti-endostatin antibody clearly demonstrated that endostatin protein was expressed in the transduced BEL-7402 cells. Extensive positive staining was observed in the cytoplasm of transduced cells (Figure 1). This indicated that human endostatin gene mediated by recombinant adenovirus was highly expressed in BEL-7402 cells and provided evidence for the feasibility of local gene therapy for HCC BEL-7402 xenografted tumors.
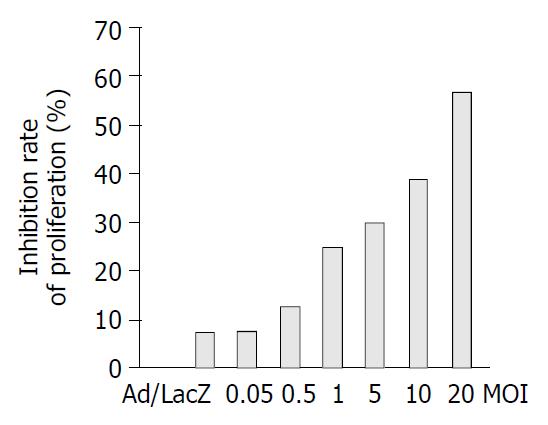
In order to investigate the effects of this secreted endostatin on proliferation of HUVEC cells, cell proliferation assays were performed by MTT assay. Ad/hEndo inhibited HUVEC cell proliferation by 7.61%, 12.61%, 24.8%, 29.86%, 38.78% and 57.2% at MOIs of 0.05, 0.5, 1.0, 5, 10 and 20, respectively. The Ad/LacZ control group showed inhibition of cellular proliferation by 7.25% at a MOI of 20 (Figure 2). These results indicated that the endostatin protein secreted by the transduced cells was highly bioactive and inhibited the proliferation of vascular endothelial cells in vitro in a dose-dependent manner, while the viral vector alone did not.
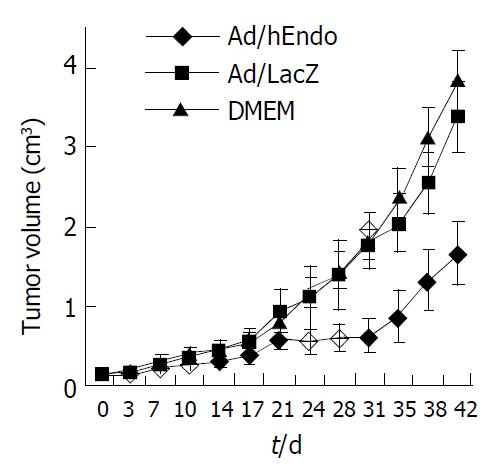
In order to observe the effect of Ad/hEndo on the growth of tumors, we established a BEL-7402 xenograft tumor model in BALB/c nude mice. After 6 wk of treatment, the growth of treated tumors was inhibited by 46.50% compared to the Ad/LacZ control group (t = 2.729, P < 0.05) and by 48.56% compared to the DMEM control group (t = 2.485, P < 0.05). The ratio of mean tumor volume in treated animals to that of the control animals (T:C ratio) was < 50% after 24 d of treatment (Figure 3). These results showed that Ad/hEndo significantly inhibited the growth of BEL-7402 xenografted tumors.
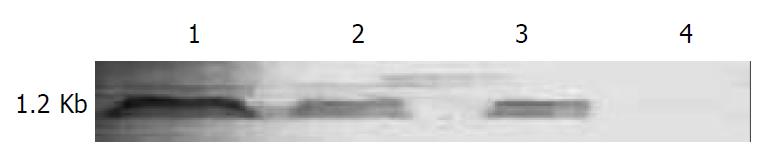
Northern blotting was performed to test whether the in vivo expressed endostatin mRNA was of full-length. After hybridization, the membrane was stained with NBT/BCIP, and endostatin mRNA was clearly demonstrated as a band of approximately 1.2 kb (Figure 4), which was the expected size of intact and functional endostatin. The highest level of endostatin mRNA in vivo was detected 1 d after intra-tumoral administration of Ad/hEndo. On d 4 and 8, endostatin mRNA levels significantly decreased. Therefore, Northern blotting showed that adenovirus-mediated human endostatin gene transfer resulted in expression of intact endostatin mRNA in vivo.
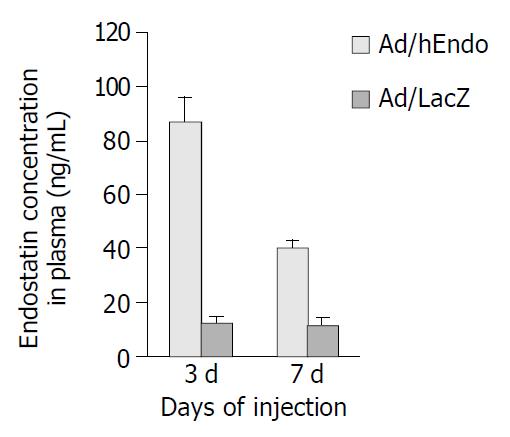
ELISA was used to measure the plasma endostatin levels. Plasma endostatin levels peaked at 87.52 ± 8.34 ng/mL on d 3 after Ad/hEndo injection, which was significantly higher than the basal level (12.23 ± 2.54 ng/mL). By d 7, plasma levels dropped to half the peak level (40.34 ± 4.80 ng/mL) (Figure 5). These results showed that intra-tumoral delivery of the endostatin gene resulted in high plasma endostatin concentrations.
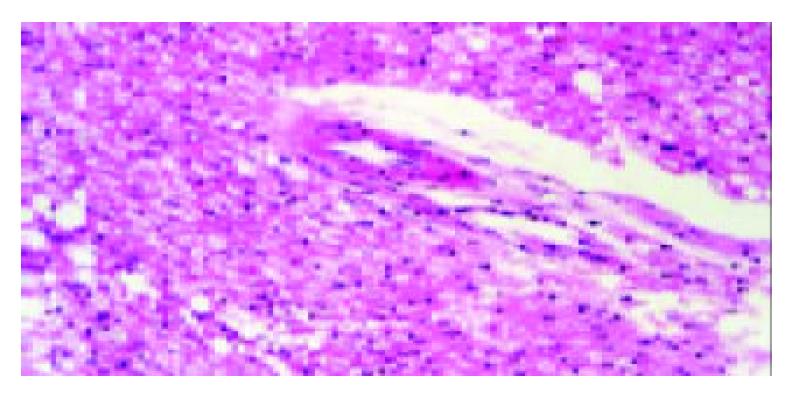
All animals were survived and appeared to be physically normal. There were no histological changes in main organs of DMEM control animals. However, histological examination in several mice of the Ad/hEndo (3/8) and Ad/LacZ (3/8) groups revealed slight to modest hepatocyte fatty degeneration, cloudy swelling and occasional necrosis companied with infiltration of lymphocyte and inflammatory cells (Figure 6). One mouse in Ad/hEndo group (1/8) and 1 in Ad/LacZ (1/8) group demonstrated slight cloudy swelling in some proximal renal tubule cells and distal renal tubule cells. No histological changes were found in spleen, lung, heart and brain of both these groups. There was no significant difference in histological changes between Ad/hEndo group and Ad/LacZ group. These results suggested that it was the recombinant adenoviruses, not endostatin protein that caused the liver and kidney toxicity.
Animal experiments on angiogenesis have frequently been conducted on tumors recently implanted in mice. However, in the human situation, tumors are often established for months or years and may possess mature vasculature that is less sensitive to anti-angiogenic therapy[1,2]. Indeed, antiangiogenesis therapies, which inhibit the growth and metastasis of neoplasms by blocking the formation of new blood vessels from existing vessels, appear to require long-term administration of the angiogenic inhibitor to ensure tumor growth suppression[3]. Endostatin protein derived from yeast (Pichia pastoris) had been introduced into clinical trials as a possible antiangiogenic tumor fighting drug[15]. The clinical studies showed that the mean half-life of recombinant human endostatin was only 10.7 ± 4.1 h in the human body, suggesting that this protein-based therapy was likely to require repeated daily or long-term administration of high-quality protein for optimal therapeutic benefits[16].
It is hoped that these difficulties of protein-based therapy may be overcome by direct expression of therapeutic gene in the body[17]. Endostatin gene therapy is a promising strategy for anti-angiogenic therapy, for a single transduction of endostatin gene could achieve a relatively long-term and high-quality protein expression in vivo[17,18]. Adenoviral vectors are commonly used as carriers for gene transfer due to their high efficiency and high capacity[19,20]. We cloned the human endostatin gene into an adenoviral shuttle plasmid and generated an adenoviral expressing system which resulted in highly efficient expression of endostatin gene and demonstrated high level of bioactivity by significantly inhibiting the proliferation of endothelial cells. In our studies, endostatin gene therapy using a recombinant adenovirus delivery significantly inhibited the growth of BEL-7402 xenografted liver tumors. Since d 24 of treatment with intra-tumoral injection with Ad/hEndo, the T:C ratio of mean tumor volume was less than 50%. After 6 courses of treatment, the growth of xenografted tumors treated with Ad/hEndo was markedly inhibited as compared with the control group.
Adenovirus can infect dividing and nondividing cells and obtain high efficiency of transgene expression[21,22]. These properties of adenoviral vector provide the possibility of local treatment, such as intra-tumoral injection and transcatheter arterial infusion of recombinant adenovirus[23]. In this study, we investigated the possibility of tumor-targeted endostatin gene therapy for liver cancer. First, we demonstrated high level transgene expression in BEL-7402 cells infected with Ad/hEndo. Then, intra-tumoral endostatin mRNA expression was clearly detected by Northern blotting on d 1 after injection with Ad/hEndo. Northern blotting indicated that the endostatin mRNA expressed inside tumor tissue was intact, thus the resultant endostatin protein could be biologically active in vivo. These positive results had provided solid evidence for local endostatin gene therapy for liver cancer.
The tumor-targeted gene therapy produced endostatin protein mostly inside the tumors[23,24], however, some of the protein entered the circulation, resulting in the peak of plasma endostatin concentration of 87.52 ± 8.34 ng/mL by d 3 post-administration. Although this plasma level was considerably lower than those reached by intravenous delivery of recombinant adenoviral vector for endostatin gene therapy[19,20], it had been shown that circulating endostatin levels of 35-40 ng/mL was sufficient to exert an antiangiogenic effect in vivo[24], suggesting that our delivery system was able to induce therapeutically relevant circulating endostatin levels.
The transient expression is a key problem for any therapeutic use[23,25], while the reason for the transient in vivo transgene expression is unclear[26,27]. Repetition of administration with therapeutic gene in vivo using a transgene vector was recommended to achieve better therapeutic effect[23,28,29]. However, multiple injections of immunogenic transgene vector will get an immune response to the vector which not only make expression even worse, but also cause systemic toxicity[30,31]. Indeed, we observed slight liver and renal damage in mice after 6 courses of endostatin gene therapy using a recombinant adenovirus delivery. Histological examination in several mice administrated with adenoviral vector revealed slight to modest hepatocyte and renal tubule cell degeneration and some degree of inflammatory reaction. It was the recombinant adenovirus, not endostatin protein that caused the liver and kidney toxicity[27,32]. Our results suggested that cautions should be taken for clinical repeated administration within a short time of the recombinant adenoviral vector carrying endostatin gene.
In conclusion, a recombinant adenoviral vector carrying human endostatin gene can be successfully constructed, with highly efficient expression of endostatin gene both in vitro and in vivo, thereby significantly inhibiting the growth of BEL-7402 xenografted liver tumors in nude mice.
Co-first-authors: Jia-Ling Huang and Qi-Cai Liu
Edited by Kumar M Proofread by Chen WW and Xu FM
| 1. | Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3254] [Cited by in RCA: 3194] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 2. | Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med. 1995;333:1757-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1649] [Cited by in RCA: 1574] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 3. | Bergers G, Javaherian K, Lo KM, Folkman J, Hanahan D. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science. 1999;284:808-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 714] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 4. | Boehm T, Folkman J, Browder T, O'Reilly MS. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature. 1997;390:404-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1201] [Cited by in RCA: 1130] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 5. | Qian J, Feng GS, Vogl T. Combined interventional therapies of hepatocellular carcinoma. World J Gastroenterol. 2003;9:1885-1891. [PubMed] |
| 6. | Li X, Feng GS, Zheng CS, Zhuo CK, Liu X. Influence of transarterial chemoembolization on angiogenesis and expression of vascular endothelial growth factor and basic fibroblast growth factor in rat with Walker-256 transplanted hepatoma: an experimental study. World J Gastroenterol. 2003;9:2445-2449. [PubMed] |
| 7. | Kim YB, Park YN, Park C. Increased proliferation activities of vascular endothelial cells and tumour cells in residual hepato-cellular carcinoma following transcatheter arterial embolization. Histopathology. 2001;38:160-166. [RCA] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3111] [Article Influence: 111.1] [Reference Citation Analysis (0)] |
| 9. | Dhanabal M, Ramchandran R, Volk R, Stillman IE, Lombardo M, Iruela-Arispe ML, Simons M, Sukhatme VP. Endostatin: yeast production, mutants, and antitumor effect in renal cell carcinoma. Cancer Res. 1999;59:189-197. [PubMed] |
| 10. | Huang X, Wong MK, Zhao Q, Zhu Z, Wang KZ, Huang N, Ye C, Gorelik E, Li M. Soluble recombinant endostatin purified from Escherichia coli: antiangiogenic activity and antitumor effect. Cancer Res. 2001;61:478-481. [PubMed] |
| 11. | Chen QR, Kumar D, Stass SA, Mixson AJ. Liposomes complexed to plasmids encoding angiostatin and endostatin inhibit breast cancer in nude mice. Cancer Res. 1999;59:3308-3312. [PubMed] |
| 12. | Ding I, Sun JZ, Fenton B, Liu WM, Kimsely P, Okunieff P, Min W. Intratumoral administration of endostatin plasmid inhibits vascular growth and perfusion in MCa-4 murine mammary carcinomas. Cancer Res. 2001;61:526-531. [PubMed] |
| 13. | Huang W, Flint SJ. The tripartite leader sequence of subgroup C adenovirus major late mRNAs can increase the efficiency of mRNA export. J Virol. 1998;72:225-235. [PubMed] |
| 14. | Huang W, Flint SJ. Unusual properties of adenovirus E2E transcription by RNA polymerase III. J Virol. 2003;77:4015-4024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Mundhenke C, Thomas JP, Wilding G, Lee FT, Kelzc F, Chappell R, Neider R, Sebree LA, Friedl A. Tissue examination to monitor antiangiogenic therapy: a phase I clinical trial with endostatin. Clin Cancer Res. 2001;7:3366-3374. [PubMed] |
| 16. | Herbst RS, Hess KR, Tran HT, Tseng JE, Mullani NA, Charnsangavej C, Madden T, Davis DW, McConkey DJ, O'Reilly MS. Phase I study of recombinant human endostatin in patients with advanced solid tumors. J Clin Oncol. 2002;20:3792-3803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 203] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Folkman J. Antiangiogenic gene therapy. Proc Natl Acad Sci USA. 1998;95:9064-9066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 143] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Szary J, Szala S. Intra-tumoral administration of naked plasmid DNA encoding mouse endostatin inhibits renal carcinoma growth. Int J Cancer. 2001;91:835-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Sauter BV, Martinet O, Zhang WJ, Mandeli J, Woo SL. Adenovirus-mediated gene transfer of endostatin in vivo results in high level of transgene expression and inhibition of tumor growth and metastases. Proc Natl Acad Sci USA. 2000;97:4802-4807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 193] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 20. | Feldman AL, Restifo NP, Alexander HR, Bartlett DL, Hwu P, Seth P, Libutti SK. Antiangiogenic gene therapy of cancer utilizing a recombinant adenovirus to elevate systemic endostatin levels in mice. Cancer Res. 2000;60:1503-1506. [PubMed] |
| 21. | St George JA. Gene therapy progress and prospects: adenoviral vectors. Gene Ther. 2003;10:1135-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 204] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 22. | Chen CT, Lin J, Li Q, Phipps SS, Jakubczak JL, Stewart DA, Skripchenko Y, Forry-Schaudies S, Wood J, Schnell C. Antiangiogenic gene therapy for cancer via systemic administration of adenoviral vectors expressing secretable endostatin. Hum Gene Ther. 2000;11:1983-1996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Kianmanesh A, Hackett NR, Lee JM, Kikuchi T, Korst RJ, Crystal RG. Intratumoral administration of low doses of an adenovirus vector encoding tumor necrosis factor alpha together with naive dendritic cells elicits significant suppression of tumor growth without toxicity. Hum Gene Ther. 2001;12:2035-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Shi W, Teschendorf C, Muzyczka N, Siemann DW. Adeno-associated virus-mediated gene transfer of endostatin inhibits angiogenesis and tumor growth in vivo. Cancer Gene Ther. 2002;9:513-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Reid T, Warren R, Kirn D. Intravascular adenoviral agents in cancer patients: lessons from clinical trials. Cancer Gene Ther. 2002;9:979-986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 183] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 26. | Molinier-Frenkel V, Le Boulaire C, Le Gal FA, Gahéry-Segard H, Tursz T, Guillet JG, Farace F. Longitudinal follow-up of cellular and humoral immunity induced by recombinant adenovirus-mediated gene therapy in cancer patients. Hum Gene Ther. 2000;11:1911-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Wen XY, Bai Y, Stewart AK. Adenovirus-mediated human endostatin gene delivery demonstrates strain-specific antitumor activity and acute dose-dependent toxicity in mice. Hum Gene Ther. 2001;12:347-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Liu Q, Muruve DA. Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther. 2003;10:935-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 323] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 29. | Geutskens SB, van der Eb MM, Plomp AC, Jonges LE, Cramer SJ, Ensink NG, Kuppen PJ, Hoeben RC. Recombinant adenoviral vectors have adjuvant activity and stimulate T cell responses against tumor cells. Gene Ther. 2000;7:1410-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Chen P, Kovesdi I, Bruder JT. Effective repeat administration with adenovirus vectors to the muscle. Gene Ther. 2000;7:587-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Pagliaro LC, Keyhani A, Williams D, Woods D, Liu B, Perrotte P, Slaton JW, Merritt JA, Grossman HB, Dinney CP. Repeated intravesical instillations of an adenoviral vector in patients with locally advanced bladder cancer: a phase I study of p53 gene therapy. J Clin Oncol. 2003;21:2247-2253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 32. | Reid T, Galanis E, Abbruzzese J, Sze D, Wein LM, Andrews J, Randlev B, Heise C, Uprichard M, Hatfield M. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dl1520): phase II viral, immunologic, and clinical endpoints. Cancer Res. 2002;62:6070-6079. [PubMed] |