Published online Jun 15, 2004. doi: 10.3748/wjg.v10.i12.1844
Revised: January 2, 2004
Accepted: January 9, 2004
Published online: June 15, 2004
Six patients infected with Cyclospora cayetanensis who sought medical care at three different hospitals in Turkey are herein presented. Four patients were male and the others were female and their ages ranged from 7 to 62 years. The first patient was HIV-positive and presented with watery diarrhea with a frequency of up to 18 times a day for more than ten months and diagnosed as cyclosporiosis in Kayseri, 1996. The second patient was also HIV positive and diagnosed as cyclosporiosis in Kayseri, 2000. The third patient was an acute myeloblastic leukemia (AML) patient and diagnosed in Istanbul, 2000. The fourth patient was idiopathic hepatic cirrhosis complaining of diarrhea and weakness and diagnosed in Kayseri, 2001. The fifth and sixth patients were immunocompetent patients complaining of diarrhea and diagnosed in Izmir and Kayseri, 2002. Diarrhea occurring from one to ten times a day continued for 7 to 70 d in the last 5 patients. Treatment with a trimethoprim/sulfamethoxazole compound was done for all patients. Both symptomatic and parasitologic improvements were quickly observed. In summary, C. cayetanensis infection is rare in Turkey and most patients infected with this pathogen tend to be immunosuppressive individuals at present.
- Citation: Yazar S, Yalcln S, Sahin &. Human cyclosporiosis in Turkey. World J Gastroenterol 2004; 10(12): 1844-1847
- URL: https://www.wjgnet.com/1007-9327/full/v10/i12/1844.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i12.1844
The genus Cyclospora is in the subclass Coccidia, phylum Apicomplexa. This genus is taxonomically related to coccidian genera in humans. This organism is a unilocular parasite previously known as cyanobacterium-like or coccidian-like body (CLB) and, in recent years, a new coccidian pathogen, Cyclospora, has been putatively identified[1,2]. The organisms are seen as nonrefractile spheres and are acid-fast variable with the modified Kinyoun’s acid-fast stains, those are unstained appear as glassy, wrinkled spheres. Modified acid-fast stains stain the oocysts from light pink to deep red, some of which may contain granules or have a bubbly appearance. The oocysts autofluoresce (strong green or intense blue) under UV epifluorescence[3]. The first known human cases of illness caused by Cyclospora infection (i.e., cyclosporiosis) were reported in the medical literature in 1979[4]. Our aim in this study was to present cases of human cyclosporiosis in Turkey and to review the literature.
We evaluated all of the human cases in our country retrospectively which were belongs to Kayseri (three cases), Istanbul (one case) and Izmir (one case) regions between 1996 and 2002[5-9]. Following these, we determined a new case with C. cayetanensis in December 2002. In this study, we discuss all of the six cases with cyclosporiosis in Turkey.
Case 1[5]
A 50 year-old woman with acquired immune deficiency syndrome (AIDS) was admitted in December 1996 for chronic diarrhea, vomiting, and fever to the Erciyes University Medical Faculty Hospital. There was preceding history of episodic watery diarrhea, vomiting, and weight loss along with intermittent fever over a period of one year. She was cachectic, with mild abdominal tenderness and alert, thrush and palpable small cervical lymph nodes. She had anemia (Hb 8.4 g/L ). Enzyme immunoassays for HIV antibodies were positive and the T4/T8 ratio was 0.6 in serum. E. coli and Proteus spp. were found at 104 cfu/mL from urine specimens. Microscopical analysis of the stool revealed numerous spherical double walled microorganisms 8-9 μm in diameter, some with internal granulation. After Kinyoun’s acid fast staining of the stool, the organisms appeared faint pink to red in colors, some cysts not taking up the stain and appearing as “ghosts’’. Empty cysts varied in shape but had generally collapsed into crescents. The organisms were identified as Cyclospora sp. The patient was treated with TMP/SMZ (160/800 mg) bid for three weeks. Following treatment, re-examination of a stool sample revealed no more organisms and diarrhea stopped. This case was the first report of Cyclospora infection in Turkey.
Case 2[6]
A 40-year old man with AIDS was evaluated parasitologically for the etiologic agent of his persistent diarrhea for two months in Erciyes University Medical Faculty Hospital in 2000. Stool samples were examined by conventional coprological methods such as fresh preparation, iodine stain, and flotation. Suspicious organisms (8-10 μm in diameter) were seen in stool, which were stained with Kinyoun’s acid-fast stain and identified as C. cayetanensis. He was treated with TMP/SMZ (160/800 mg) bid for two weeks. We could not find the organism in the stool samples after the treatment and diarrhea stopped.
Case 3[7]
In 2000, at the Istanbul University Medical Faculty Hospital there was another 7 year-old male patient with acute myeloblastic leukemia (AML). A sudden diarrhea developed while bone narrow transplantation was being planned. The patient’s stool samples were examined with respect to pathogen bacteria and fungi and rotavirus. None of them was determined, isolated and/or seen. Two stool specimens, which were taken in one-week interval, also were examined with modified trichrome, acid fast and safranin staining methods. In microscopic examinations, C. cayetanensis oocysts were seen with all three staining methods. After four weeks of therapy with trimethoprim-sulphamethoxazole (15 mg/kg.d) diarrhea stopped and in the new stool specimens, C. cayetanensis oocysts were not encountered.
Case 4[8]
A 52 year-old male patient with idiopathic hepatic cirrhosis complaining of diarrhea and weakness was accepted to the gastroenterology clinic of Erciyes University Medical Faculty Hospital in 2001. In the patient’s history, there were watery, bad smelling, bloodless episodes of diarrhea, fever, cold, sweating, and a 10 kg lost of weight which all began three weeks prior to hospitalization. The patient had never traveled to a foreign country. Physical examination did not reveal any abnormalities except subicteric conjunctivas and a hyperemic tongue. The patient was apyretic. In laboratory examination, blood values were found as Hb: 14.2 g/dL, white blood cells: 4 900/mm³, platelets: 69 000/mm³. Biochemical values were as follows: K: 2.9 mmol//L (↑), P: 1.7 mg/dL (↑), Ca: 7.6 mg/dL (↑), Mg: 0.9 mg/dL (↑), uric acid: 9.3 mg/dL (↑), total bilirubin: 4.2 mg/dL (↑), AST: 165 U/L (↑), ALT: 76U/L (↑), CK-MB: 120 U/L (↑), albumin: 2.8 gr/dL (↑), acetone, protein and bilirubin were (+) in urine. Anti-HBs Ag and Anti-HAV were positive and other hepatitis markers were negative. Anti-HIV antibodies were found to be negative by ELISA test. In abdominal USG, liver with lobule contour was smaller than normal size, and its echo was increased. Widespread intraperitoneal exudate was seen.
In order to find out the causative etiologic agent of diarrhea, stool samples were examined by different methods and stained using modified Kinyoun’s acid-fast stain. Following examination, acid-fast variable wrinkled spheres approximately 9 µm in diameter, were seen and diagnosed as C. cayetanensis. Confirmation of the diagnosis was established by fluorescent microscope (380 to 420 nm excitation filter), which showed bright green to intense blue autofluorescent oocysts.
Consequently, this organism was diagnosed as C. cayetanensis. The patient was treated with TMP/SMZ (160/800 mg) bid for 7 d. Following treatment, re-examination of a stool sample, however, did not reveal the presence of any organisms.
Case 5[9]
A 30 year-old female patient complaining of diarrhea and weakness was admitted to the gastroenterology clinic of Atatürk State Hospital in I zmir, 2002. In the patient's history, there were watery diarrhea, fever, nausea which all began one week prior to admission to hospital. Stool samples were examined by conventional coprological methods such as fresh preparation, iodine stain, flotation, modified Ritchie’s method and modified Kinyoun’s acid-fast stain. Acid-fast variable wrinkled spheres were seen and diagnosed as oocysts of C. cayetanensis. Confirmation of the diagnosis was established by fluorescent microscope. After one-week therapy with trimethoprim-sulphamethoxazole for 7 d, diarrhea stopped and in the new stool specimens, C. cayetanensis oocysts were not seen. This patient was different from the other four cases in Turkey because there were no abnormalities in immunologic tests of the patient. That is, she was an immunocompetent patient.
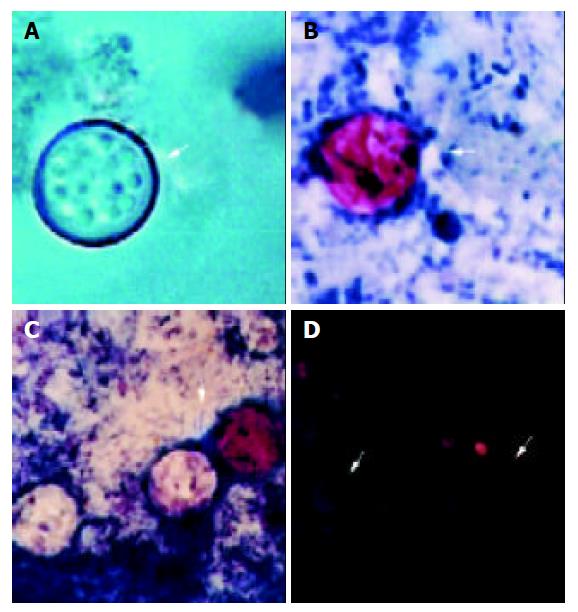
A 62-year-old male patient complaining of diarrhea was admitted to the Erciyes University Medical Faculty Hospital in October 2002. This case was not reported anywhere. In the patient’s history, there were watery, bad smelling diarrhea, fever, sweating, which all began one week before admission to hospital. Stool samples were examined by conventional coprological methods. Acid-fast variable oocysts were seen and diagnosed as oocysts of C. cayetanensis (Figure 1).
After one-week therapy with trimethoprim-sulphamet-hoxazole (160/800 mg) bid for 7 d diarrhea stopped and in the new stool specimens, C. cayetanensis oocysts were not encountered and all the symptoms disappeared. This patient was also immunocompetent similar to case 5. There were no abnormalities in immunologic tests of the patient.
Cyclospora organisms were first reported in the intestines of moles in 1870 by Eimer, and Schneider introduced the genus Cyclospora in 1881. The first report of human Cyclospora infection came from Papua New Guinea in 1979[4].
Cyclospora oocysts in freshly excreted stool are non-infectious. The oocysts are thought to require days to weeks outside the host under favorable environmental conditions to sporulate and thus to become infectious. Direct person-to-person transmission by fecal exposure is unlikely, because excreted oocysts must sporulate to become infective[10].
C. cayetanensis constitutes a significant cause of chronic and intermittent diarrhea in immunocompromised patients especially those with AIDS. A study in Haiti has documented the occurrence of chronic diarrhea in most patients with AIDS[11]. Cyclospora infection has also been reported in patients with severe AIDS in other areas[12,13]. Lontie et al[14] reported 2 cases of intestinal Cyclospora infection in immunocompetent Belgians. In Turkey, there were 6 cases between 1996 and 2002, 2 of them were AIDS patients[5,6], 1 of them was an AML patient[7], 1 of them was idiopathic hepatic cirrhosis[8] and the last two patients were immunocompetent individuals[9,10].
Both epidemiological and environmental data suggested that the organism be waterborne[1]. The transmissibility of Cyclospora through water depends on the probability that the water source of interest could become contaminated and that the water treatment would kill or remove oocysts. Cyclospora oocysts, like Cryptosporidium oocysts, probably are highly chlorine resistant, but they could be more easily removed by conventional filtration because they are about twice as big as Cryptosporidium oocysts[10].
In 1994, an outbreak of Cyclospora occurred among British soldiers and dependents stationed in a small military base detachment in Pokhara, Nepal. That outbreak was epidemiologically linked to drinking water, because the organism was identified in the water source[15].
In the 1990s, at least 11 definite and probable food born outbreaks of cyclosporiosis, affecting at least about 3 600 people, were documented, all of which occurred in North America[10]. The outbreak that brought cyclosporiosis to prominence in North America and definitively established that Cyclospora was transmissible through food, occurred in 1996 in the United States and Canada and was linked to a third country, Guatemala, which was the source of implicated fresh raspberries[16].
The symptoms presenting in one patient (watery diarrhea, nausea, weight loss, and abdominal pain) were similar to those classically Cyclospora infection[11,13,17]. Since the oocysts of Cyclospora are acid-fast like those of Cryptosporidium, we recommend that all laboratories screening for the latter parasite include precise measurements of oocysts. Cyclospora oocysts are 8-10 µm in diameter (intermediate size between
Cryptosporidium parvum and Isospora belli). Cryptosporidium parvum oocyts have an average width and length of 4.5 µm 5 µm, respectively. It is possible that many cases of diarrhea reported to be due to Cryptosporidium might actually be due to Cyclospora because size discriminations are not often made. Cyclospora organisms have now been isolated in chronic diarrhea and this infection should be carefully distinguished from cryptosporidiosis[1,11,18]. A list of some of the similarities and differences between these two organisms is shown in Table 1.
| Similarities | C. cayetanensis | C. parvum |
| Acid-fast staining of oocysts | Variable acid-fast | Acid-fast |
| Number of infective units | 4 | 4 |
| (sporozoites) per sporulated oocyst | ||
| Completion of life cycle within humans | Yes, except for sporulation | Yes |
| Multiplication outside the host (e.g. in water or food) | No | No |
| Differences | ||
| Size of oocysts | 8-10 µm in diameter (intermediate | Average width of 4.5 µm and |
| in size between Cryptosporidium | average length of 5 µm | |
| parvum and Isospora belli) | ||
| Number of organisms in stools of | Typically excreted in low | Often excreted in somewhat |
| symptomatic nonimmune hosts | to moderate number | higher numbers |
| Autofluorescence of oocyst wall | Yes | No |
| Internal morphology of sporulated oocysts | Each oocyst has 2 internal | The 4 sporozoites are naked |
| sporocysts, each contains 2 sporozoites | within the oocyst | |
| Infectivity of oocysts in freshly excreted stool | Must sporulate outside host to | Fully sporulated and infectious |
| become infectious | when excreted (sporozoites can | |
| be visualised when oocysts are excreted | ||
| Zoonotic potential | Host range unknown | Infects virtually all commonly known |
| wild and domestic mammals | ||
| Location in enterocytes of small bowel | Intracytoplasmic within | Intracellular, extracytoplasmic, within |
| a parasitophorous vacuole | a parasitophorous vacuole at luminal | |
| in apical supranuclear region | surface of enterocyte | |
| Susceptibility to antimicrobial agents | Treatment with TMP-SMZ leads to | Some antimicrobial agents |
| both clinical and parasitologic cure | (e.g. paromomycin) may cause clinical | |
| improvement, but no agents has been | ||
| consistently demonstrated to provide | ||
| parasitologic cure |
The treatment for Cyclospora infections cotrimoxasole (TMP-SMZ) was given for 7-10 d (longer, if symptoms persist)[19]. The adult dosage was 160 mg TMP plus 800 mg SMZ orally twice daily. In a double blind, placebo controlled trial among Peruvian children, a three-day course of TMP (5 mg/kg.d) plus SMZ (25 mg/kg.d) decreased the duration of oocyst excretion, but few symptomatic children were treated to address the effect on duration of diarrhea[20]. Alternative treatments have not yet been identified. Limited data suggest that the following drugs are ineffective: albendazole, azithromycin, nalidixic asid, norfloxacin, tinidazole, metronidazole, quinacrine, tetracycline, and diloxanide furoate. Approaches to alternative treatment of patients who could not tolerate TMP-SMZ therapy include observation and symptomatic treatment[21-25]. In a small, randomized, controlled-trial comparing oral TMP-SMZ and ciprofloxacin for treatment of and secondary prophylaxis for Cyclospora infection in HIV infected Haitians, ciprofloxacin (500 mg twice daily for 7 d as therapy and thrice weekly for 10 wk as secondary prophylaxis) was moderately effective, though it was less active than TMP-SMZ[26]. These results suggest that ciprofloxacin might be an alternative for patients who cannot tolerate TMP-SMZ. However, these results should be confirmed in a large number of patients as well as in non-HIV population.
Sporadic cases of infection may be part of widespread outbreaks and should in any case be reported to public health officials. Pubic health personals and clinicians should also be aware that stool examination for Cyclospora should be specifically required in case of clinical suspicion of Cyclospora infection (protracted or relapsing diarrheal episode)[10]. In conclusion, this organism should be considered in the differential diagnosis of unexplained diarrhea in both immunosuppressive and immunocompetent patients. However, further studies are needed to confirm the causative association with other diseases and to determine the incidence and epidemiological features of this organism.
Edited by Wang XL and Xu FM
| 1. | Long EG, Ebrahimzadeh A, White EH, Swisher B, Callaway CS. Alga associated with diarrhea in patients with acquired immunodeficiency syndrome and in travelers. J Clin Microbiol. 1990;28:1101-1104. [PubMed] |
| 2. | Ortega YR, Sterling CR, Gilman RH, Cama VA, Díaz F. Cyclospora species--a new protozoan pathogen of humans. N Engl J Med. 1993;328:1308-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 209] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Garcia SL, Bruckner AD. Intestinal protozoa: Coccidia and Microsporidia in Diagnostic medical parasitology. Washington, D. C: American Society Microbiol 1997; 54-89. |
| 4. | Ashford RW. Occurrence of an undescribed coccidian in man in Papua New Guinea. Ann Trop Med Parasitol. 1979;73:497-500. [PubMed] |
| 5. | Koc AN, Aygen B, Sahin Ý, KayabasÜ . Cyclospora sp. associ-ated with diarrhea in a patient with AIDS in Turkey. Tr J Med Scien. 1998;28:557-558. |
| 6. | Yazar S, Aygen B, Koc AN, Altunoluk B, Alp E, Sahin I. A diarrheal case caused by Cyclospora cayetanensis: Case report. Erciyes Med J. 2000;22:48-51. |
| 7. | Büget E, Büyükbaba BÖ, Klrkoyun UH, Aglrbasll H, Yalman N, Anak S, Can E, Gedikoglu G. Türkiye'de ilk kez belirlenen Cyclospora cayetanensis etkenli diyare olgusu. J Turks Mic Soc. 2000;30:162-165. |
| 8. | Yazar S, Yaman O, Demirtaş F, Yalçin S, Yücesoy M, Sahin I. Cyclospora cayetanensis associated with diarrhea in a patient with idiopathic compensated hepatic cirrhosis. Acta Gastroenterol Belg. 2002;65:241-244. [PubMed] |
| 9. | Türk M, Türker M, Ak M, Karaayak B, Kaya T. Cyclosporiasis associated with diarrhoea in an immunocompetent patient in Turkey. J Med Microbiol. 2004;53:255-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Herwaldt BL. Cyclospora cayetanensis: a review, focusing on the outbreaks of cyclosporiasis in the 1990s. Clin Infect Dis. 2000;31:1040-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 169] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Pape JW, Verdier RI, Boncy M, Boncy J, Johnson WD. Cyclospora infection in adults infected with HIV. Clinical manifestations, treatment, and prophylaxis. Ann Intern Med. 1994;121:654-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 173] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Madico G, Gilman RH, Miranda E, Cabrera L, Sterling CR. Treatment of Cyclospora infections with co-trimoxazole. Lancet. 1993;342:122-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Hart AS, Ridinger MT, Soundarajan R, Peters CS, Swiatlo AL, Kocka FE. Novel organism associated with chronic diarrhoea in AIDS. Lancet. 1990;335:169-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Lontie M, Degroote K, Michiels J, Bellers J, Mangelschots E, Vandepitte J. Cyclospora sp.: a coccidian that causes diarrhoea in travellers. Acta Clin Belg. 1995;50:288-290. [PubMed] |
| 15. | Rabold JG, Hoge CW, Shlim DR, Kefford C, Rajah R, Echeverria P. Cyclospora outbreak associated with chlorinated drinking water. Lancet. 1994;344:1360-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Herwaldt BL, Ackers ML. An outbreak in 1996 of cyclosporiasis associated with imported raspberries. The Cyclospora Working Group. N Engl J Med. 1997;336:1548-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 191] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Albert MJ, Kabir I, Azim T, Hossain A, Ansaruzzaman M, Unicomb L. Diarrhea associated with Cyclospora Sp. in Bangladesh. Diagn Microbiol Infect Dis. 1994;19:47-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Soave R. Cyclospora: an overview. Clin Infect Dis. 1996;23:429-435; quiz 436-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Hoge CW, Shlim DR, Ghimire M, Rabold JG, Pandey P, Walch A, Rajah R, Gaudio P, Echeverria P. Placebo-controlled trial of co-trimoxazole for Cyclospora infections among travellers and foreign residents in Nepal. Lancet. 1995;345:691-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 92] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Madico G, McDonald J, Gilman RH, Cabrera L, Sterling CR. Epidemiology and treatment of Cyclospora cayetanensis infection in Peruvian children. Clin Infect Dis. 1997;24:977-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 61] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Soave R, Herwaldt BL, Relman DA. Cyclospora. Infect Dis Clin North Am. 1998;12:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Shlim DR, Cohen MT, Eaton M, Rajah R, Long EG, Ungar BL. An alga-like organism associated with an outbreak of prolonged diarrhea among foreigners in Nepal. Am J Trop Med Hyg. 1991;45:383-389. [PubMed] |
| 23. | Shlim DR, Pandey P, Rabold JG, Walch A, Rajah R. An Open Trial of Trimethoprim Alone against Cyclospora Infections. J Travel Med. 1997;4:44-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Connor BA, Shlim DR, Scholes JV, Rayburn JL, Reidy J, Rajah R. Pathologic changes in the small bowel in nine patients with diarrhea associated with a coccidia-like body. Ann Intern Med. 1993;119:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Wurtz R. Cyclospora: a newly identified intestinal pathogen of humans. Clin Infect Dis. 1994;18:620-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Verdier RI, Fitzgerald DW, Johnson WD, Pape JW. Trimethoprim-sulfamethoxazole compared with ciprofloxacin for treatment and prophylaxis of Isospora belli and Cyclospora cayetanensis infection in HIV-infected patients. A randomized, controlled trial. Ann Intern Med. 2000;132:885-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |