Published online Jun 15, 2004. doi: 10.3748/wjg.v10.i12.1769
Revised: February 14, 2004
Accepted: February 21, 2004
Published online: June 15, 2004
AIM: To investigate Time- and pH-dependent colon-specific drug delivery systems (CDDS) for orally administered diclofenac sodium (DS) and 5-aminosalicylic acid (5-ASA), respectively.
METHODS: DS tablets and 5-ASA pellets were coated by ethylcellulose (EC) and methacrylic acid copolymers (Eudragit® L100 and S100), respectively. The in vitro release behavior of the DS coated tablets and 5-ASA coated pellets were examined, and then in vivo absorption kinetics of DS coated tablets in dogs were further studied.
RESULTS: Release profile of time-dependent DS coated tablets was not influenced by pH of the dissolution medium, but the lag time of DS release was primarily controlled by the thickness of the coating layer. The thicker the coating layer, the longer the lag time of DS release is. On the contrary, in view of the pH-dependent 5-ASA coated pellets, 5-ASA release was significantly governed by pH. Moreover, the 5-ASA release features from the coated pellets depended upon both the combination ratio of the Eudragit® L100 and S100 pH-sensitive copolymers in the coating formulation and the thickness of the coating layer. The absorption kinetic studies of the DS coated tablets in dogs demonstrated that in vivo lag time of absorption was in a good agreement with in vitro lag time of release.
CONCLUSION: Two types of CDDS, prepared herein by means of the regular coating technique, are able to achieve site-specific drug delivery targeting at colon following oral administration, and provide a promising strategy to control drug release targeting the desired lower gastrointestinal region.
- Citation: Cheng G, An F, Zou MJ, Sun J, Hao XH, He YX. Time- and pH-dependent colon-specific drug delivery for orally administered diclofenac sodium and 5-aminosalicylic acid. World J Gastroenterol 2004; 10(12): 1769-1774
- URL: https://www.wjgnet.com/1007-9327/full/v10/i12/1769.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i12.1769
Currently, a novel oral colon-specific drug delivery system (CDDS) has been developing as one of the site-specific drug delivery systems. This delivery system, by means of combination of one or more controlled release mechanisms, hardly releases drug in the upper part of the gastrointestinal (GI) tract, but rapidly releases drug in the colon following oral administration. The necessity and advantage of CDDS have been well recognized and reviewed recently[1-3]. In view of CDDS specifically delivering drug to the colon, a lot of benefits would be acquired in terms of improving safety and reducing toxicity when treating local or systemic chronic diseases. First, as for treating localized colonic diseases, i.e. ulcerative colitis, Crohn’s disease and constipation etc., the optimal drug delivery system, such as CDDS, should selectively deliver drug to the colon, but not to the upper GI tract[1]. For this reason, the drug concentration was significantly lessened in the upper GI tract, while increased considerably in the colon, resulting in alleviated GI side effects. Second, the colon is referred to as the optimal absorption site for protein and polypeptide after oral administration, because of the existence of relatively low proteolytic enzyme activities and quite long transit time in the colon[2,3]. To our knowledge, CDDS could provide reliable protection against GI enzymatic degradation by releasing the polypeptide and protein nearly unchanged and fully efficacious in the preferred colon, thereafter resulting in remarkably increased bioavailability for protein and polypeptide. Finally, CDDS would be advantageous when a delay in absorption is desirable from a therapeutical point of view, as for the treatment of diseases that have peak symptoms in the early morning and that exhibit circadian rhythms, such as nocturnal asthma, angina and rheumatoid arthritis[1,4,5].
There were currently a few strategies to achieve colonic specificity, such as bacterially triggered and pressure-controlled CDDS[6]. The aim of this study was to explore the feasibility of the time- and pH-dependent CDDS, diclofenac sodium (DS) and 5-aminosalicylic acid (5-ASA) being selected as model drugs, respectively. Besides, we were intended to exploit the typical pharmaceutical coating technology to attain the time-and pH-dependent colon-specific drug delivery. Time-dependent colon-specific DS coated tablets consisted of a tablet core and a coating layer composed of a water-insoluble ethylcellulose (EC) and a water-soluble channeling agent. pH-dependent colon-specific 5-ASA coated pellets consisted of a pellet core and a coating layer of the pH-sensitive methacrylic acid copolymers (Eudragit® L100 and S100).
DS was frequently used for treating rheumatoid arthritis[2], which had apparent circadian rhythms and peak symptoms in the early morning. When orally administering DS conventional formulation, it was difficult to achieve the desired clinical effect, because it elicited patients’ incompliance of administration in the early morning to coordinate the rhythm of rheumatoid arthritis, due to rapid absorption of the conventional formulation. However, colon-specific DS delivery was not only effective, but also more convenient for administration than the conventional formulation. On the other hand, 5-ASA was usually used to effectively treat ulcerative colitis and Crohn’s disease in clinical practice[7]. It was unstable in the stomach, and readily absorbed in the small intestine, eliciting the undesirable adverse effect. Thereby, 5-ASA was prepared as the CDDS in this study, which protected 5-ASA from the upper GI conditions[1] and allowed for 5-ASA rapid release in the designated colonic region.
Preparation of DS core tablets Core tablets consisted of 40% (w/w) diclofenac sodium (Yanzhou Pharm., China), 40% (w/w) sodium carboxymethyl starch sodium, 8% (w/w) sodium chloride, 2% (w/w) other excipients. Drug and excipients were blended and sieved (mesh 80); the mixtures were granulated with adhesives and dried 1 h at 55 °C. Core tablets with average weight of 100 mg were prepared using a single punch tablet machine (Type-TDP, 1st Pharm Machine Manu. Co., Shanghai, China). The diameter of core tablets was 6 mm.
Tablet coating The core tablets were coated in a conventional rotating pan. For the coating process, the rotation speed was adjusted to 36 r/min with the coating pan set at an angle of 45°, nozzle port size 0.8 mm; inlet air temperature was 35 °C; tablet bed temperature was 25 °C; the coating solution was sprayed onto the tablets at a flow rate of 0.8 mL/min. The coating solution was 30 g/L EC (Colorcon Co., China) in ethanol solution which contained diethyl phthalate (20% of EC, w/w) as plasticizer, and PEG 400 (15% of EC, w/w, Pharm. Med. Supply Ltd. Co., China) as channeling agent.
Dissolution test Dissolution studies were carried out with paddle method at a rotation speed of 100 r/min and 900 mL distilled water as medium at 37 °C (n = 6). Samples were collected at predetermined time points, analyzed for DS contents using a UV-spectrophotometer (UV-9100, Ruili Co., China) at 276 ± 1 nm and calculated cumulative release amounts of DS over the sampling times.
Experimental protocol Six male dogs (weight 20 ± 2.5 kg) were randomly assigned to one of two crossover experiments with a 7-d washout period. Dogs were fasted overnight for 12 h before administration and free access to water was allowed. Each dog was orally administered either reference formulation (four enteric coated tablets, Liaodong Pharm. Ltd. Co., batch: 010910, tablet containing 25 mg of DS) or test formulation (four EC coated tablets, each tablet containing 25 mg of DS with 7.5% coating level), respectively. Blood samples were collected at predetermined times for each protocol, respectively: (1) 1, 2, 2.5, 3, 3.5, 4, 4.5, 5, 6, 7, 8, 10, and 24 h for the reference; (2) 1, 3, 4, 5, 5.5, 6, 6.5, 7, 7.5, 8, 9, 10, 12, and 24 h for the test. Plasma was immediately obtained by centrifuging the blood samples at 3 000 r/min for 10 min. The plasma samples were maintained in a freezer at -20 °C until analysis.
Chromatographic conditions The quantitative determination was performed on a high-performance liquid chromatograph equipped with a PU-1580 pump (Jasco, Japan) and 970/975 UV detector (Jasco, Japan). The column was a Hypersil ODS C-18 (250 mm×4.6 mm, 5 μm). The mobile phase consisted of methanol: water: triethylamine: glacial acetic acid (68:22: 0.044:0.044, v/v)[8]. The eluate was monitored at 274 nm, with a sensitivity of 0.001 AUFS. A flow rate was 1.0 mL/min, and the column temperature was maintained at 35 °C.
Sample preparation A 0.5 mL plasma sample was mixed with 20 μL of 4.8 mmol/L inuprofen (internal standard) and 0.6 mL of 1.0 mol/L phosphate buffer in a glass centrifuge tube. The solution was mixed on a vortex mixer for 2 min, and then added 3 mL of a solution of hexane and isopropyl alcohol (4:1, v/v). The mixture was mixed on a vortex mixer for 2 min again and centrifuged at 3 000 r/min for 5 min. The organic layer was transferred into a clean tube and evaporated to dryness under nitrogen at 35 °C. The residue was dissolved in 100 μL of mobile phase and 20 μL of the solution were injected into the HPLC system.
Data analysisCmax represents the maximum drug concentration, tmax the time to reach peak concentration, and tlag the lag time of drug. These values were obtained as directly measured values. The elimination rate constant (Ke) was calculated from the slope of the logarithm plasma concentration to time at the end four points. The areas under the plasma concentration-time curve (AUC0-tn) were calculated by the trapezoidal method. The relative bioavailability (F %) was calculated by the equation: F(%) = (AUCTest formulation·DoseReference formulation)/(AUCReference formulation·Dose Test formulation). The percentages of absorption in vivo were calculated by the Wagner-Nelson method.
Preparation of 5-ASA pellets Pellets (1.0-1.2 mm in diameter) consisted of 5-aminosalicylic acid (5-ASA, Genlou Chem. Ltd. Co., China), microcrystalline cellulose (MCC, Avicel PH101, Asahi Chem. Ind. Ltd. Co., Japan) and lactose. Drug and excipients were blended and sieved (mesh 80); the mixtures were prepared as wet mass with adhesives. Pellets were prepared by extruder and spheronizer (Institute of Machinery for Chemical Industry East China University of Science and Technology, Shanghai, China). The speed of the extrusion was 30 r/min. The speed of the spheronization was 1 200 r/min and the time of the spheronization was 5 min[9,10].
Pellet coating The pellets were coated in a fluidized-bed coating apparatus (Shenyang Pharm. Univer., China). For the coating process, nozzle port size was 0.8 mm; inlet air temperature 45 °C; atomizing pressure 1.2 kg/cm2; coating solution was sprayed onto the pellets at a flow rate of 0.8 mL/min. The coating solution was aqueous dispersions of various proportions of Eudragit® L100 and Eudragit® S100 (Röhm Pharma GmbH, Germany). The plasticizer TEC was added directly to the polymer dispersions[11].
Dissolution test Dissolution studies were carried out with a basket method at a 100 r/min rotation speed and 37 °C, 900 mL dissolution medium according to ChP 2000. The dissolution tests were performed in HCl pH1.2, phosphate buffer solution pH6.0, pH6.8, pH7.2 or pH7.5, respectively. Samples were collected at predetermined time points, analyzed for 5-ASA content using a UV-spectrophotometer (UV-9100, Ruili Co., China) at 302 nm and 330 nm for 5-ASA assay in 0.1 mol/L HCl and the phosphate buffer solutions, respectively.
In order to simulate the pH changes along the GI tract, three dissolution media with pH1.2, pH6.0, and pH7.2, were sequentially used, referred to as the sequential pH change method (Table 1). pH6.0, and pH7.2 media were 0.05 mol/L phosphate buffer solution. When performing release experiments, the pH1.2 medium was first used for 2 h, then removed, and the fresh pH6.0 dissolution medium was added. After 1 h, the medium was removed again, and fresh pH7.2 dissolution medium added. The UV readings were taken at 302 nm and 330 nm for 5-ASA measurement in 0.1 mol/L HCl and the buffer media, respectively[12,13]. And then the 5-ASA cumulative release percentage was calculated over the sampling times.
| pH | λmax (nm) | Time (h) | Simulated GI region |
| 1.2 | 302 | 2 | Stomach |
| 6.0 | 330 | 1 | Duodenum |
| 7.2 | 330 | 5 | Lower small intestine and colon |
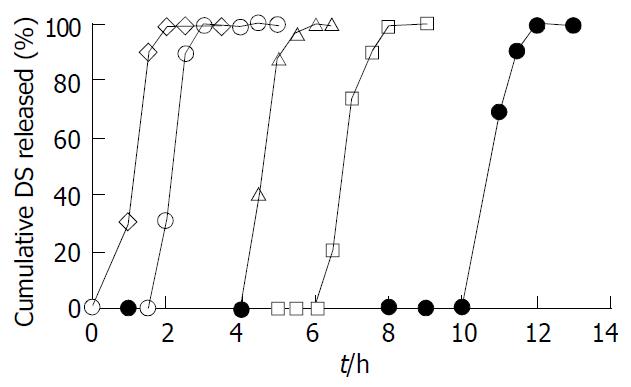
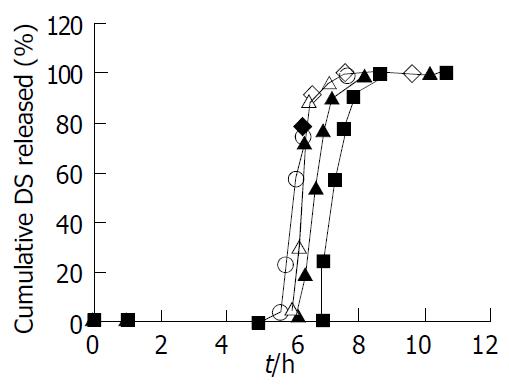
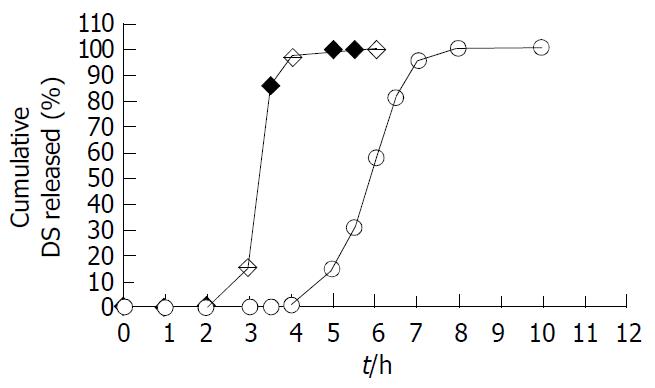
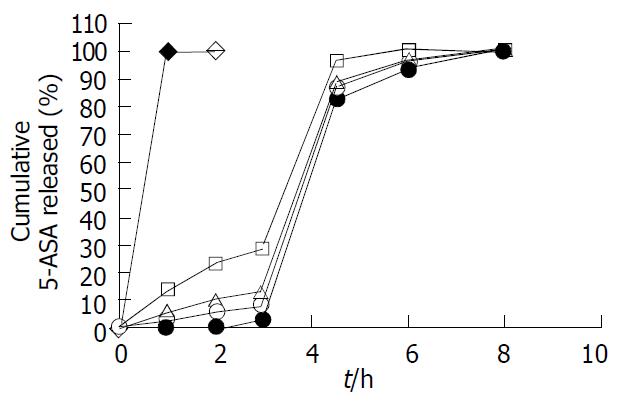
The DS core tablets were coated by the coating formulation, consisting of 30 g/L EC, diethyl phthalate (20% of EC, w/w) as a plasticizer and PEG 400 (15% of EC, w/w) as a channeling agent in ethanol solution. The in vitro release behavior of the DS coated tablets with different coating levels (3%, 5%, 7%, 9%, and 11%, w/w), was studied. As shown in Figure 1, the coating level exercised a significant effect on the lag time of DS release from the coated tablets. Manifestly, the greater the coating level, the longer the lag time of DS release from the coated tablets. However, once the coating layer was broken up, DS then was rapidly released from the coated tablets (Figure 1). The effects of dissolution medium pH on the release profiles of the DS coated tablets at 7.5% coating level are shown in Figure 2. To be evident, the pH values of media did not exert a significant effect on the DS release pattern from the coated tablets. The in vitro release profile of test DS coated tablets at 7.5% coating level was compared with the marketed DS enteric tablets, as shown in Figure 3.
The reference enteric tablets started DS release at 2 h, while the test DS coated tablets began to release DS at 4 h, after being put in the dissolution medium. The lag time of DS release from the test tablets was significantly longer than that of reference enteric tablets. Moreover, the cumulative DS release percentage of test tablet was nearly 100% within 7 h.
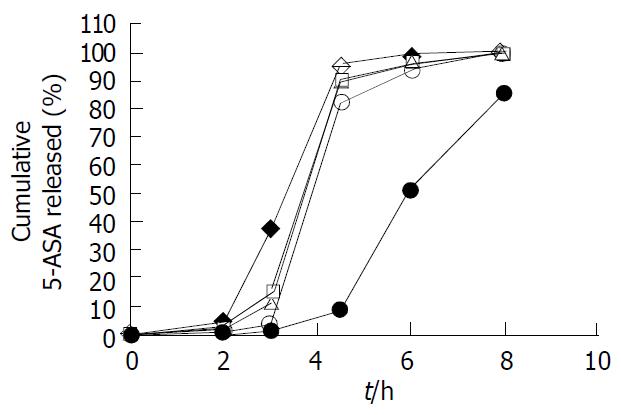
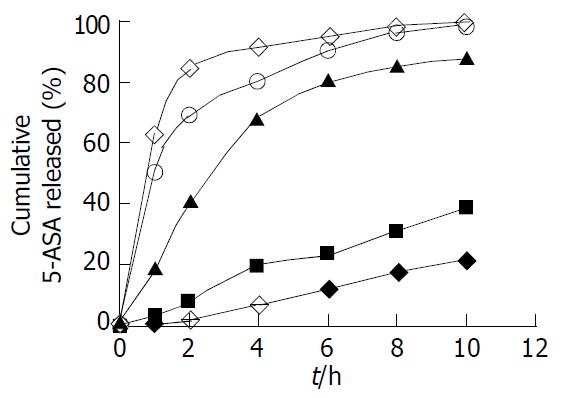
The 5-ASA pellets were coated with combination of Eudragit® L100-Eudragit® S100 methacrylic acid pH-sensitive copolymers, and their release characteristics were examined by three sequential dissolution media with different pH values in order to mimic pH changes along GI tract. We investigated the effect of combination ratios of Eudragit® L100-Eudragit® S100 in the coating formulation on the release profiles of 5-ASA coated pellets, as shown in Figure 4.
The combination ratios of Eurdragit® L100-Eudragit® S100 in the coating formulation played a significant role in regulating the release behavior of 5-ASA coated pellets. When employing 1:4 (w/w) combination ratio of Eudragit® L100-Eudragit® S100 as the coating formulation, 5-ASA was released less than 1.0% in pH1.2 dissolution medium (simulated gastric fluid) until 2 h, and less than 3.0% in pH6.0 (simulated duodenum fluid) at 1 h after changed to fresh medium, and more than 80% in pH7.2 (simulated lower small intestinal and colonic fluid) at 1.5 h after changed to fresh medium. When the pellets were coated with Eudragit® L100-Eudragit® S100 combination (w/w) ratios of 1:0, 4:1, and 1:1, the cumulative release percentage of 5-ASA was 1.0%-3.6% in pH1.2 until 2 h, 11%-37% in pH6.0 at 1 h, and more than 80% in pH7.2 at 1.5 h, the results indicating that the earlier measurable release occurred. Pellets coated with Eudragit® S100 alone displayed the slowest release rate, the cumulative release percentage of 5-ASA was less than 0.5% in pH1.2 up to 2 h, was less than 1.0% in pH6.0 at 1 h, and was not more than 10% in pH7.2 at 1.5 h.
The effects of the coating level on the release properties of 5-ASA coated pellets were also examined, with the coating level ranging from 0% to 25% and the combination ratio of Eudragit® L100-Eudragit® S100 set at 1:4 (w/w), as shown in Figure 5. As the coating level and thickness of coating layer increased, the acid-resistant property of coating layer was enhanced. The coating level being 25% (w/w), almost none of the 5-ASA was released from coated pellets in pH1.2 and pH6.0 dissolution media. By contrast, the cumulative release percentage of 5-ASA was more than 80% in pH7.2 at 1.5 h, regardless of the coating levels. Accordingly, this formulation, with the combination ratio Eudragit® L100-Eudragit® S100 being 1:4 (w/w) and the coating level being 25%, would be a promising candidate for designed DS pH-dependent CDDS.
Moreover, we examined the influences of medium pH values on the release characteristics of the 5-ASA coated pellets, utilizing 1:4 (w/w) combination ratio Eudragit® L100-Eudragit® S100 as the coating formulation and setting the coating level at 25%. Five pH values (1.2, 6.0, 6.8, 7.2 and 7.5) of dissolution media were utilized. As shown in Figure 6, pH values of medium notably affected 5-ASA release profiles from the pH-dependent coated pellets. When pH increased from 1.2 to 7.5, the release rate of 5-ASA from the coated pellets was outstandingly enhanced.
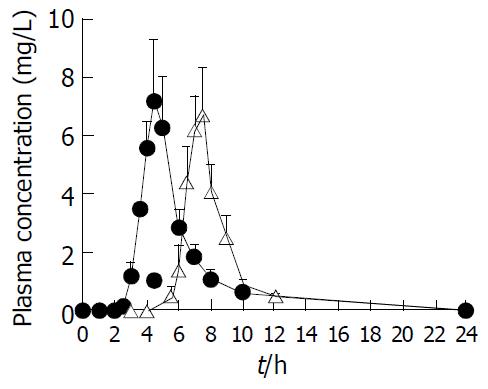
Either DS reference formulation (four 25 mg enteric coated tablets) or DS test formulation (four 25 mg EC coated tablets) was orally administered in the two crossover experimental design in dogs at a dose of 100 mg/body, respectively. The concentration-time curve of DS in plasma is illustrated in Figure 7. The pharmacokinetic parameters are calculated and listed in Table 2. The mean lag time of DS absorption from the test formulation was as long as 5.80 h, while that for the reference formulation was 2.80 h after oral administration. This result was well consistent with the mean in vitro lag time of DS release from two formulations, the test tablets being 4 h versus reference enteric tablets being 2 h. In addition, Cmax of the test and the reference formulations was 8.17 mg/L and 10.05 mg/L, respectively. The relative bioavailability of test formulation versus reference formulation was 86.38%.
| Parameter | Test formulation | Reference formulation |
| Cmax (mg/L) | 8.17 ± 1.35 | 10.05 ± 1.66 |
| tmax (h) | 7.40 ± 0.35 | 4.50 ± 0.20 |
| Ke (L/h) | 0.56 ± 0.06 | 0.44 ± 0.11 |
| tlag (h) | 5.80 ± 0.42 | 2.80 ± 0.21 |
| AUC0-tn (mg·h/L) | 17.21 ± 2.09 | 19.91 ± 2.83 |
| F (%) | 86.38 ± 10.51 | — |
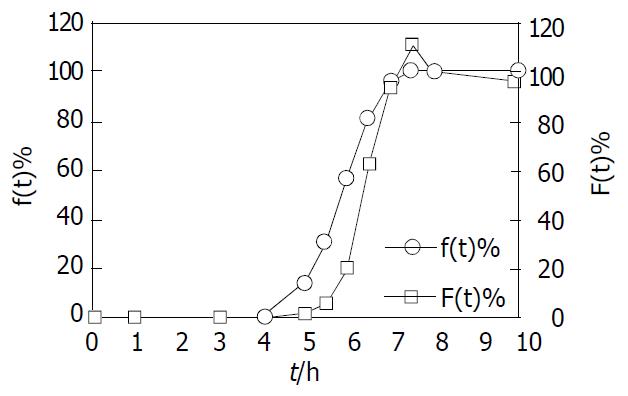
Absorption percentage in vivo of DS for test formulation was assessed by the Wagner-Nelson method. Both absorption percentage in vivo of DS (F(t)) and the cumulative release percentage in vitro of DS (f(t)) versus time profiles, demonstrate a similar contour curve in Figure 8. Furthermore, there existed a significant correlation between F(t) and f (t) (r = 0.95) for test formulation, indicative of the good relationship between in vitro release and in vivo absorption processes.
It has been well found that gastric emptying of a preparation was variable and depended primarily upon whether subjects were fed or not, and upon the properties of the preparation. On the contrary, the transit time in small intestine for the preparation was surprisingly constant at 3 ± 1 h and appeared to be independent of the types of the preparations and whether subjects were fed or not[14]. On the basis of the above understandings, we designed the time-dependent colon-specific drug delivery to achieve no measurable drug release in the upper part of GI tract and a considerable drug release after arrival at colon.
The time-dependent DS coated tablets were prepared by means of the coating technology, with the coating formulation consisting of the water-insoluble EC, the water-soluble channeling agent PEG 400 and the membrane plasticizer diethyl phthalate. Generally, the coating layer of EC was mainly hydrophobic and was moderately impermeable to water molecules. And the water-soluble channeling agent was rapidly dissolved in water, resulting in the formation of numerous micro-channels across the coating layer. Then, water molecules could penetrate into the tablet core via these channels to cross the hydrophobic EC membrane. Water uptake caused the expansion of the disintegrating agent within the tablet core, resulting in a gradual build-up of pressure within the coated tablets. Once the inside pressure exceeded the critical disrupted value that the coating layer resisted most, the coating layer would be ruptured and release drug in a manner of burst, thus forming the delay effect of drug release from the coated tablets. Taken together, the formation of micro-channels was the first crucial step, and the critical disrupted pressure governed by the coating layer and the expansibility of the disintegrating agent were the key factors for time-dependent controlled release[15].
The release profiles of the DS coated tablets at 7.5% coating level were not affected by the pH values of media (Figure 2). This was reasonably expected, since the channeling agent PEG 400 was pH-insensitive and formation of the micro-channels was not affected by pH. The effects of the coating level on the lag time of DS release from the coated tablets were tested, indicating that the coating level played a significant role in controlling the lag time of release. To increase the coating level would extend the lag time of DS release from the coated tablets (Figure 1). There were two possible mechanisms contributing to this phenomenon. For one thing, since the hydrophobic EC membrane was relatively impermeable to water molecules, substantial effect on the rate of the water molecules diffusion and water uptake, was exerted by the number, size, length and tortuosity of the aqueous micro-channels in the coating layer. The coating level and thickness of the coating layer certainly determined the rate of water uptake, by controlling the formation rate and physical characteristics of the aqueous channels. To be evident, the increase of the coating level would elicit the longer and more tortuous aqueous micro-channels, severely impairing the rate of water uptake. Alternatively, the thicker coating layer developed the higher critical resistant pressure to be surpassed for disrupting the coating membrane, required the greater water uptake to gradually build up inside pressure by the disintegrating agent’s expansion and prolonged the lag time of drug release from the coated tablets[16].
The mean in vivo lag time of the DS absorption from coated tablets was significantly longer than that from enteric tablets, being 5.80 h vs 2.80 h (Figure 7 and Table 2). The results were in accordance with the in vitro lag time of DS release from two formulations, and the lag time of DS release from test tablets was nearly 2 h longer than that from the enteric tablets (Figure 3). Furthermore, the in vivo absorption kinetics of the time-dependent DS coated tablets was assessed by the typical Wagner-Nelson method. The absorption percentage in vivo versus time profile was analogous to the cumulative release percentage in vitro versus time profile, which validated the in vitro dissolution conditions and verified the utility of the time-dependent CDDS (Figure 8). The good correlation coefficient (r = 0.95) between in vivo absorption and in vitro release percentages, further confirmed this conclusion.
To our knowledge, there exists a pH gradient along the GI tract, a fact that pH in the stomach is approximately 1.5 in the fasted state, ranges roughly from 5.0 to 7.0 in the small intestine, and from 6.0 to 7.2 in the colon[1]. The pH values at the end of ileum and in the colon are notably higher than the upper GI tract, therefore providing the rationale for developing pH-dependent CDDS. In addition, a novel dosage form, pellets, is the multiple units’ drug delivery system, and it is superior to the conventional preparation, such as tablets, for the following reasons. The pellets appear to be less influenced by the physiologic factors, such as the gastric emptying, intestinal transit, than tablets. Moreover, the pellets could be widely and evenly distributed in the GI tract surfaces, which increase the drug-tract contact surface and thus improve bioavailability. Finally, the release failure of the individual unit hardly affects the total release behavior due to the multiple units, whereas tablets do not[17].
Eudragit® L100 and S100 were methacrylic acid pH-sensitive copolymers and began to be dissolved from pH6.0 and pH7.0, respectively. The combination utilization of L100 and S100 would be capable of designing the suitable copolymers intended to begin being dissolved from the desired pH environment in the lower GI tract (pH variation range of 6.0-7.0). Clearly, the combination ratio of L100 and S100 exerted a great effect on the 5-ASA release characteristic from the coated pellets. The combination ratios (1:0, 4:1, 1:1, and 1:4, 0:1) of L100 and S100 hardly released drug in pH1.2 until 2 h, indicating the acid-resistant properties of the coated polymers (Figure 4). After changing to pH6.0 fresh medium, L100 could to be dissolved and the channels were then quickly created in the coating membrane; conversely, S100 did not. Therefore, the higher the proportion of L100 in the coating formulation, the more channels formed, thus allowing for the higher 5-ASA release[12]. After converting into the pH7.2 fresh medium, both L100 and S100 could be dissolved; by contrast, the dissolution rate of L100 was faster than S100. Thus the higher amount of L100 in the coating formulation, the faster dissolution and disappearance of the coating layer, leading to rapid release of 5-ASA (Figure 4). The dissolution profile for the 0:1 coating formulation at pH7.2 demonstrated that the dissolving process of the coating layer of S100 alone was rather slow, resulting in very slow drug release.
Fixing the combination ratio of Eudragit® L100 and S100 at 1:4, the coating level remarkably influenced the 5-ASA release from coated pellets (Figure 5). When the coating level was less than 25%, a measurable 5-ASA release was observed in pH1.2 and 6.0 media. The coated pellets at 25% coating level scarcely released 5-ASA in pH1.2 and 6.0 media, and rapidly released in pH7.2 medium. This coating level of pellets (25%) was significantly higher than that of tablets (10%) in this study, because of the ratio of surface area of pellets considerably greater than tablets. The lower coating level for pellets would form an incomplete closed membrane around pellets, and elicited a measurable drug release in pH1.2 and 6.0 media (Figure 5). The coating level of pellets 25%, however, could form uninterrupted and homogenous coating layer, swiftly released 5-ASA in pH7.2, but not in pH1.2 and 6.0 media. On the other hand, the higher coating level would require the longer processing time, escalation of cost and further 5-ASA was incapable of being rapidly released from pellets in pH7.2 medium (the coating level ≥ 35%, unpublished data). Since the coating copolymers were pH-sensitive, 5-ASA release from the coated pellets was expected to be influenced by the dissolution medium pH, as confirmed in Figure 6.
In summary, two controlled release mechanisms, i.e. time-and pH-dependent, could achieve colonic-specific drug delivery following oral administration. In addition, both CDDS were relatively inexpensive and easy to be manufactured using conventional pharmaceutical coating technique, and provided the promising candidates for specifically delivering drug to the targeted colon region, in particular for DS and 5-ASA in this study, respectively.
Edited by Zhang JZ and Chen WW Proofread by Xu FM
| 1. | Kinget R, Kalala W, Vervoort L, van den Mooter G. Colonic drug targeting. J Drug Target. 1998;6:129-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 126] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Watts PJ, lllum L. Colonic drug delivery. Drug Dev Ind Pharm. 1997;23:893-913. [RCA] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Yang L, Chu JS, Fix JA. Colon-specific drug delivery: new approaches and in vitro/in vivo evaluation. Int J Pharm. 2002;235:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 332] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 4. | Halsas M, Penttinen T, Veski P, Jürjenson H, Marvola M. Time-controlled release pseudoephedrine tablets: bioavailability and in vitro/in vivo correlations. Pharmazie. 2001;56:718-723. [PubMed] |
| 5. | Halsas M, Hietala J, Veski P, Jürjenson H, Marvola M. Morning versus evening dosing of ibuprofen using conventional and time-controlled release formulations. Int J Pharm. 1999;189:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Zou MJ, Cheng G. The oral colon-specific drug delivery system. Shenyang Yaoke Daxue Xuebao. 2001;18:376-380. |
| 7. | Ardizzone S, Bianchi Porro G. A practical guide to the management of distal ulcerative colitis. Drugs. 1998;55:519-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Miller RB. High-performance liquid chromatographic determination of diclofenac in human plasma using automated column switching. J Chromatogr. 1993;616:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Pinto JF, Buckton G, Newton JM. The influence of four selected processing and formulation factors on the production of spheres by extrusion and spheronisation. Int J Pharm. 1992;83:187-196. [RCA] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Krogars K, Heinämäki J, Vesalahti J, Marvola M, Antikainen O, Yliruusi J. Extrusion-spheronization of pH-sensitive polymeric matrix pellets for possible colonic drug delivery. Int J Pharm. 2000;199:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Khan MZ, Prebeg Z, Kurjaković N. A pH-dependent colon targeted oral drug delivery system using methacrylic acid copolymers. I. Manipulation Of drug release using Eudragit L100-55 and Eudragit S100 combinations. J Control Release. 1999;58:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 189] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Khan MZ, Stedul HP, Kurjaković N. A pH-dependent colon-targeted oral drug delivery system using methacrylic acid copolymers. II. Manipulation of drug release using Eudragit L100 and Eudragit S100 combinations. Drug Dev Ind Pharm. 2000;26:549-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Ashford M, Fell JT, Attwood D, Woodhead PJ. An in vitro inves-tigation into the suitability of pH-dependent polymers for co-lonic targeting. Int J Pharm. 1993;91:241-245. [RCA] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 106] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Rouge N, Buri P, Doelker E. Drug absorption sites in the gas-trointestinal tract and dosage forms for site-specific delivery. Int J Pharm. 1996;136:117-139. [RCA] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 145] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Xiao YC, Cheng G, Zou MJ. Studies on coated theophylline tab-lets for colon-specific delivery using Eudragit NE 30D. Shenyang Yaoke Daxue Xuebao. 2001;18:5-7. |
| 16. | Fan TY, Wei SL, Yan WW, Chen DB, Li J. An investigation of pulsatile release tablets with ethylcellulose and Eudragit L as film coating materials and cross-linked polyvinylpyrrolidone in the core tablets. J Control Release. 2001;77:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Lu B, Wang PY, Deng YJ, Zhou Q, Ni D, Xu HN, Xu HL, Liang WQ, Pei YY, Wei SL. New techniques and new dosage forms of drug. 1th ed. Beijing: People’s Medical Publishing House 1998; 289-305. |