INTRODUCTION
Colonic epithelium, which lines the surface of both crypts and villi, plays an important role in the maintenance of water and electrolyte balance. While it is well established that colonic fluid secretion is driven by electrogenic colonic Cl- secretion[1], HCO3- secretion has also been shown to be important in the human colon. In many diarrhea disorders, a high concentration of HCO3- was frequently found in stool water and patients with severe diarrhea often suffered from metabolic acidosis due to sustained HCO3- losses in the stool[2]. However, the contribution of crypts and villus epithelia to colonic Cl- and HCO3- secretion is not known. Although it has been generally believed that colonic villus (or surface) epithelium is mainly involved in NaCl and water absorption while crypt epithelium is involved in secretion[1,3]. There is now clear evidence that electrolyte secretion is located in both[1,4]. The cystic fibrosis transmembrane conductance regulator (CFTR), a cAMP-dependent Cl- channel known to mediate both Cl-[5] and HCO3- secretion[6,7], has been shown to be present in both crypt and villus epithelial cells although higher expression level of CFTR was found in crypts than in villi[8,9]. Human colonic cell lines have been derived to provide useful models in studying electrolyte transport properties of colonic crypts and villus epithelial cells. T84 cell line is a well-characterized colonic epithelial model that maintains a secretory phenotype similar to crypt base cells, and has been widely used to examine the regulation of intestinal Cl- secretion since CFTR and Na+-K+-Cl- cotransporter (NKCC) are highly expressed in this cell line[1,10,11]. The properties of human colonic Caco-2 cells are mostly close to those of mature absorptive villus/surface cells but they possess a certain CFTR characteristic of secretory cells[1,12,13]. However, few studies have been conducted to compare the secretory properties of these cell lines.
Tetramethylpyrazine (TMP, also named ligustrazine), a compound purified from Ligustium Wollichii Francha, is a widely used active ingredient in Chinese herbal medicine for the treatment of cardiovascular diseases due to its vasodilatory actions and antiplatelet activity[14-16]. TMP has been proposed to act as an inhibitor of phosphodiesterase (PDE) and thereby it increases intracellular cAMP[15]. We have recently demonstrated a stimulatory effect of a TMP-containing herbal formula on the anion secretion in human colonic[17] and pancreatic duct cell lines[18], indicating the involvement of cAMP and activation of CFTR. The present study aimed to compare the stimulatory effects of TMP on colonic anion secretion in different colonic cell types, T84 and Caco-2, as well as freshly isolated rat colonic mucosa, in an attempt to assess the different contribution of these two cell types to colonic anion secretion.
MATERIALS AND METHODS
Chemicals and solutions
Tetramethylpyrazine was purchased from Beijing Fourth Pharmacy (Beijing, China). Diphenylamine-2, 2’-dicarboxylic acid (DPC) was obtained from Riedel-de Haen Chemicals (Hannover, Germany). Amilorode hydrochloride was obtained from Sigma Chemical Company (St. Louis, MO). Calbiochem (San Diego, CA) was the source for 4, 4’-diisothiocyanostilbene-2, 2’-disulfonic acid (DIDS), bumetanide, tetrodotoxin (TTX), indomethacin and glybenclamide. Dulbecco’s modified Eagle’s medium (DMEM)/F12, Hanks’ balanced salt solution (HBSS) and fetal bovine serum were from Gibco Laboratory (New York, NY). Krebs-Henseleit solution (K-HS) had the following compositions (mmol/L): NaCl, 117; KCl, 4.5; CaCl2, 2.5; MgCl2, 1.2; NaHCO3, 24.8; KH2PO4, 1.2; glucose, 11.1. The solution was gassed with 950 mL/L O2 and 50 mL/L CO2, and kept the pH at 7.4. In some experiments gluconate was used to replace anions Cl- or Cl-/HCO3- for making a Cl- free or Cl-/HCO3- free K-HS. For Cl-/HCO3--free K-HS, HEPES and Tris were used and the solution was gassed with absolute O2.
Cell culture
Human colonic T84 and Caco-2 cells were purchased from American Type Culture Collection (Rockville, MD). The cells were routinely maintained in DMEM/F12 for T84 or in DMEM for Caco-2 with 100 mL/L fetal bovine serum, 100 kU/L penicillin and 100 mg/L streptomycin. The cells were fed 3 times a week, and (2-3)×105 cells were plated on to the floating permeable support, which was made of a Millipore filter with a silicone rubber ring attached on top of it for confining the cells (0.45 cm2). Cultures were incubated at 37 °C in 950 mL/L O2-50 mL/L CO2 for 4-5 d before experiments.
Colonic mucosa preparation
Adult male Sprague-Dawley rats (Laboratory Animal Services Center, the Chinese University of Hong Kong) ranging in age from 8 to 12 wk had free access to standard rodent laboratory food and water until the day of the experiments. The animals were killed by exposure to absolute CO2. Segments of distal colon about 8 cm proximal to the anus were quickly removed, cut along the mesenteric border into flat sheets and flushed with ice-cold K-HS bubbled with 950 mL/L O2-50 mL/L CO2. The tissues were pinned flat with the mucosal side down in a Sylgard-lined petri dish containing ice-cold oxygenated solution. The serosa, submucosa, and muscular layer were stripped away with fine forceps to obtain a mucosa preparation which was pretreated with indomethacin (10 µmol/L), a synthesis inhibitor of prostaglandins (PG), and 1 µmol/L of tetrodotoxin (TTX), a blocker of neuron sodium channel, 30 min before treatment with TMP. Three or four of these stripped mucosal preparations were obtained from each animal.
Short-circuit current measurements
The measurement of ISC was described previously[19]. Between the two halves of the Ussing chamber, in which the total cross-sectional area was 0.45 cm2, monolayers of cell lines grown on permeable supports were clamped and flat sheets of rat stripped colonic mucosa were mounted, which were bathed in both sides with K-HS and maintained at 37 °C by a water jacket enclosing the reservoir. K-HS was bubbled with 950 mL/L O2-50 mL/L CO2 to maintain the pH of the solution at 7.4. Drugs could be added directly to apical or basolateral side of the epithelium. Transepithelial potential difference for every monolayer or colonic mucosa was measured by the Ag/AgCl reference electrodes (World Precision Instrument, USA) connected to a preamplifier that was in turn connected to a voltage-clamp amplifier DVC-1000 (World Precision Instrument, USA). In most of the experiments, the change in ISC was defined as the maximal rise in ISC following agonist stimulation and it was normalized to current change per unit area of the epithelial monolayer (µA/cm2). The total charge transported for 15 min (the area under the curve of the agonist-induced ISC response) was also used to describe the agonist-induced response (μC/cm2). Experiments were normally repeated in different batches of culture to ensure that the data were reproducible.
Reverse transcription PCR (RT-PCR) analysis
Total RNA (15 µg) was extracted from T84, Caco-2 cells and rat colonic mucosa. Human pancreatic duct epithelial cells, CAPAN-1, were used as a positive control. Expression of NBC was analyzed by competitive RT-PCR. The specific oligo nucleotide primers for NBC were CCT CAG CTC TTC ACG GAA CT for sense and AGC ATG ACA GCC TGC TGT AG for antisense corresponding to nucleotides 333-949 with expected cDNA of 616 bp[20]. Internal marker, GAPDH was used for semi-quantitative analysis of NBC expression in Caco-2 and T84 cells. The specific oligo nucleotide primers for GAPDH were TCC CAT CAC CAT CTT CCA G for sense and TCC ACC ACT GAC ACG TTG for antisense corresponding to nucleotides 249-764 bp with expected cDNA of 515 bp[20].
Statitical analysis
Results were expressed as mean ± SE. The number of experiments represented independent measurements on separate monolayers. Comparisons between groups of data were made by either the Student’s t-test (2-group comparison) or one-way ANOVA with Newman-Keuls post-hoc test (3-group comparison). A P value less than 0.05 was considered statistically significant. EC50 values were determined by nonlinear regression using GraphPad Prism software.
RESULTS
TMP- induced ISC responses in T84 and Caco-2 cells
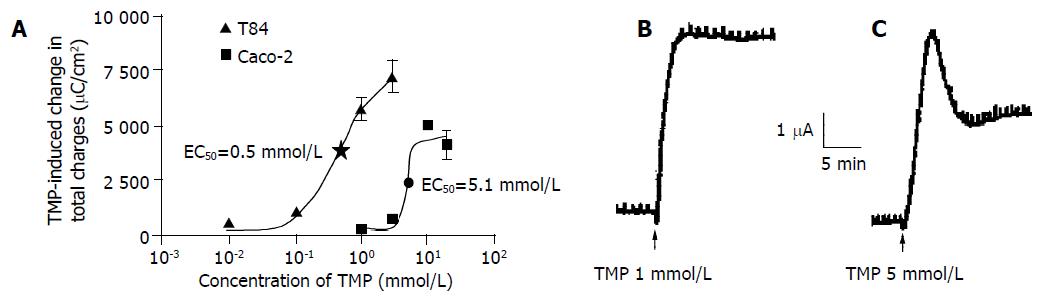
TMP stimulation increased ISC in both T84 and Caco-2 cells when the drug was added to either the apical or basolateral membrane. However, greater responses were achieved when TMP was added to apical membrane of T84 and basolateral membrane of Caco-2. The TMP-induced responses were concentration-dependent with an apparent EC50 at 0.5 and 5.1 mmol/L for T84 and Caco-2 cells respectively (Figure 1A). The TMP-induced changes in ISC were calculated as the total charge transported for 15 min (the area under the curve of the TMP-induced ISC response for the given time period) since the current kinetics was different in the two cell types. TMP produced a fast and sustained increase of ISC in T84 cells with averaged total charge of 6100 ± 451 μC/cm2 (n = 8, Figure 1B) transported over 15 min in response to TMP at a concentration close to corresponding EC50 (1 mmol/L). However, TMP produced a ISC response in Caco-2 cells with a fast transient peak followed by a lower but sustained plateau with averaged total charge of 2 293 ± 214.7 μC/cm2 (n = 7) in response to TMP at corresponding EC50 (5 mmol/L, Figure 1C). Similar TMP-induced ISC characteristics were observed in the same cell type at all concentrations of TMP used.
Figure 1 TMP-induced ISC response in T84 and Caco-2 cell lines.
The concentration-response curve for TMP-induced response in T84 and Caco-2 cells, and each datum was obtained from at least 4 individual experiments. A: Values are mean ± SE of maximal ISC increase; B: Representative ISC recordings in response to apical addition of TMP (1 mmol/L) in T84 cells; C: Basolateral application of TMP (5 mmol/L) in Caco-2 cells.
Involvement of Cl- and HCO3- in TMP-induced ISC responses in colonic cell lines
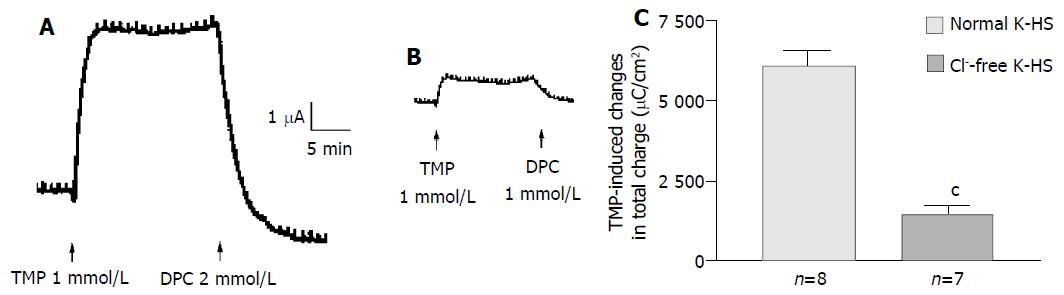
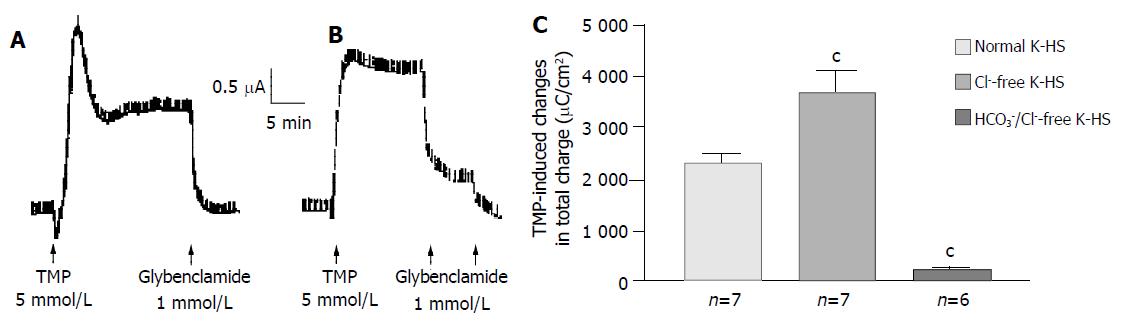
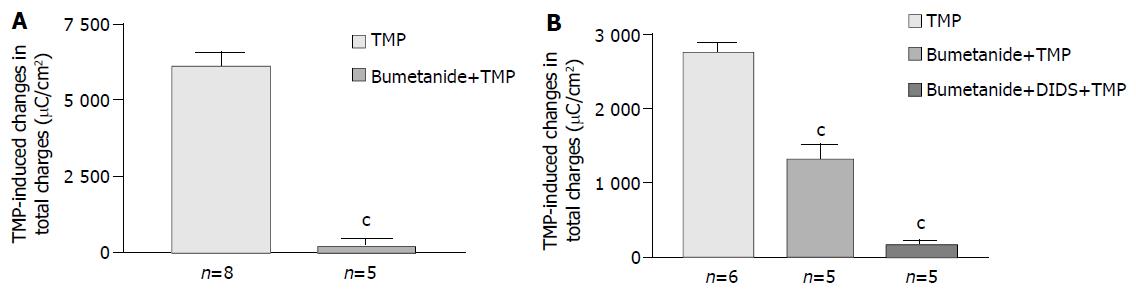
Removal of Cl- from the bathing solution inhibited TMP-induced ISC increase by 76%, from 6 100 ± 451 µC/cm2 to 1 375 ± 103 µC/cm2 in T84 cells (n = 7, P < 0.001, Figure 2). In Caco-2 cells the TMP-induced current in Cl--free solution was not reduced, but rather increased to 3 700 ± 388 µC/cm2 (n = 6, P < 0.001, Figure 3). However, the TMP-induced ISC in Caco-2 was reduced by 90%, to 228.9 ± 27.6 µC/cm2 (n = 6, P < 0.001) when both extracellular Cl- and HCO3- were removed (Figure 3). Apical addition of Cl- channel blockers, DPC (2 mmol/L) or glibenclamide (1 mmol/L), could completely abolish the TMP-induced response in T84 (Figure 2A,B) and Caco-2 cells (Figure 3A,B). Basolateral addition of Na+-K+-2Cl- cotransporter (NKCC) inhibitor, bumetanide (100 µmol/L), inhibited TMP-induced ISC in T84 cells by 96.7%, from 6 100 ± 451 µC/cm2 (n = 8) to 200 ± 71 µC/cm2 (n = 5, P < 0.001, Figure 4A), but only 47.9% in Caco-2 cells, from 2 720 ± 144 µC/cm2 (n = 6) to 1 304 ± 200 µC/cm2 (n = 5, P < 0.001, Figure 4B). However, when an inhibitor of NBC, DIDS (200 µmol/L), was combined with bumetanide, it reduced TMP-induced current in Caco-2 cells to 181 ± 41 µC/cm2 by 93.3% (n = 5, P < 0.001, Figure 4B). Pretreatment with epithelial Na+ channel (ENaC) blocker, amiloride (10 µmol/L) (n = 5, P > 0.05) or removal of Na+ from apical solution (n = 4, P > 0.05) did not significantly affect the TMP-induced ISC (data not shown), indicating that TMP-produced responses were mediated by Cl-/HCO3- secretion, but not Na+ absorption.
Figure 2 Cl- dependence of TMP-induced ISC increase in T84 cells.
Representative ISC recording with arrows indicating TMP (1mmol/L) added apically, which was blocked by apical adding DPC. Values are mean ± SE; cP < 0.001. A: Normal; B: Cl-free; C: K-HS, comparison of TMP-induced total charge transferred in normal and Cl--free K-HS.
Figure 3 HCO3- dependence of TMP-induced ISC increase in Caco-2 cells.
Representative ISC recording with arrows indicating TMP (5 mmol/L) added basolaterally, which was abolished by apical application of glybenclamide. Values are mean ± SE; aP < 0.05, bP < 0.01 and cP < 0.001. A: Normal; B: Cl-free; C: K-HS, comparison of TMP-induced total charges transferred in normal and Cl--free K-HS.
Figure 4 Effect of inhibitors of basolateral anion transporters on TMP-induced ISC responses.
Values are mean ± SE; cP < 0.001. A: Comparison of TMP (1 mmol/L)-induced ISC responses in T84 cells in the absence and presence of basolateral addition of bumetanide (100 μmol/L); B: Comparison of TMP (5 mmol/L) -induced ISC responses in Caco-2 cells in the absence and presence of basolateral addition of inhibitors indicated.
TMP-induced ISC response in colonic mucosa
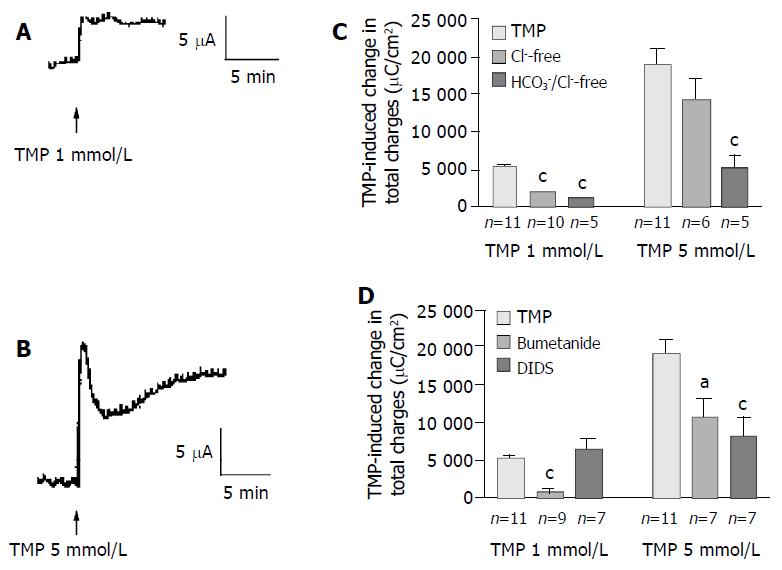
In freshly isolated rat colonic mucosa, TMP induced different ISC responses depending on the concentration used. One mmol/L of TMP evoked a sustained ISC increase (Figure 5A) similar to that observed in T84 cells, with averaged total charge of 5 133 ± 465 µC/cm2 (Figure 5C, n = 11). However, at the concentration greater than 5 mmol/L, TMP induced a ISC response similar to that observed in Caco-2 cells, a transient peak followed by a more sustained phase (Figure 5B), with averaged total charge of 18 945 ± 2 023 µC/cm2 (Figure 5C, n = 11). Removal of extracellular Cl- inhibited 65.5% of the ISC induced by 1 mmol/L TMP (Figure 5C, n = 10, P < 0.001). However, the ISC induced by 5 mmol/L of TMP was not significantly reduced by Cl- removal (n = 6, P > 0.05) but was reduced by 71.6% in Cl- and HCO3- -free solution (Figure 5, n = 5), indicating a greater extent of the involvement of HCO3-in the response to 5 mmol/L TMP as compared to the response
Figure 5 TMP-induced anion secretions in rat colonic mucosa.
Representative ISC recording values are mean ± SE; aP < 0.05, bP < 0.01, cP < 0.001. A: 1 mmol/L; B: 5 mmol/L of TMP added basolaterally in normal K-HS; C: Comparison of TMP (1 and 5 mmol/L)-induced ISC obtained in normal and Cl--free as well as both of Cl- and HCO3- -free solutions; D: Comparison of TMP (1 and 5 mmol/L)-induced ISC obtained in basolateral pretreatment of colonic mucosa with bumetanide (100 µmol/L) and DIDS (100 µmol/L).
RT-PCR analysis of NBC expression in T84, Caco-2 cells and colonic mucosa
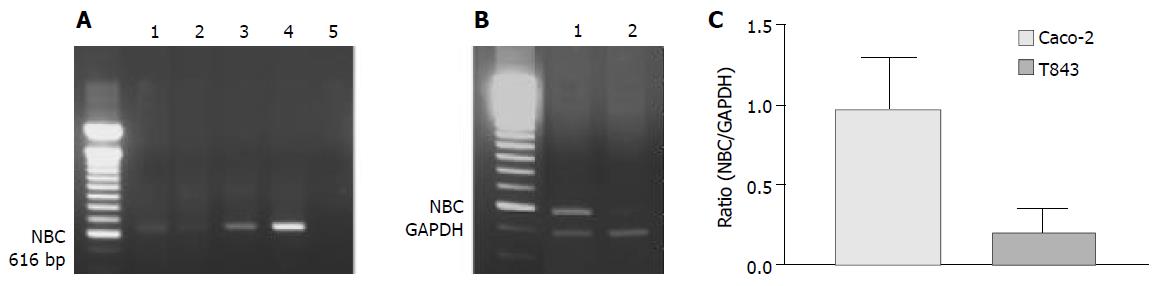
NBC was expressed in all cells and tissues examined by RT-PCR (Figure 6A). However, semi-quantitative analysis showed that the nucleotide expression level in Caco-2 cells was 4.8-fold over that of T84 (Figure 6B). The NBC nucleotide fragment was confirmed by nucleotide sequencing (Figure 6C).
Figure 6 RT-PCR analysis of mRNA expression of Na+- HCO3- cotransporter (NBC) in colonic cells.
A: RT-PCR results with products as expected of NBC found in rat colon (1), T84 (2) and Caco-2 (3) with positive control (4) using pancreatic duct cells (CAPAN-1) and negative control (5) where cDNA was omitted. B: Semi-quantitative analysis of NBC expression in Caco-2 (1) ; C: T84 (2) cells with NBC to GAPDH (internal marker) ratio shown.
DISCUSSION
The present study has demonstrated for the first time the effects of TMP on the colonic anion secretion and revealed that the different doses of TMP could induce differential Cl- and HCO3-mediated anion secretion by different colonic cell types. The differential effects of TMP on T84 and Caco-2 cells were not only reflected by the differences in the concentration-dependent ISC responses and the ISC kinetics, but also in the extents to which Cl- and HCO3- were involved in mediating the TMP responses. This was supported by the observation that the TMP-induced response in T84 was sensitive to extracellular Cl- replacement or an inhibitor of basolateral NKCC, bumetanide, while the response in Caco-2 cells was relatively insensitive to these treatments, at least to a much less extent. Instead, the response in Caco-2 cells was sensitive to the removal of extracellular HCO3- and an inhibitor of basolateral NBC, which has been shown to mediate HCO3- entry into the cells in a number of tissues including the pancreas[21,22], intestine[23], kidney[24] and endometrium[25]. Although DIDS is also known to inhibit Cl-/HCO3- exchanger (AE), the involvement of AE in this case was unlikely since it was not electrogenic. The high expression of NBC in Caco-2 cells but not T84 cells was also consistent with the DIDS sensitivity profiles in the two cell types, suggesting the involvement of NBC in mediating basolateral HCO3- transport in Caco-2 cells. The observed predominant Cl- secretion elicited by TMP in T84 cells was consistent with the Cl--secreting characteristics of this colonic crypt-like cell model. Although secretory properties were less well characterized in Caco-2 cells, a previous study[26] has demonstrated ISC increases in response to cAMP-evoking agents including PDE inhibitor, 3-isobutyl-1-methylxanthine (IBMX), in Caco-2 cells with biphasic current kinetics and DIDS sensitivity similar to that of the TMP-induced ISC response observed in the present study. Interestingly, in the same study alkalization of the apical solution could be induced which was also inhibited by basolateral addition of DIDS. Similar HCO3--dependent and DIDS-sensitive ISC was also observed in the intestine[27-29]. Together with the presently demonstrated NBC expression in Caco-2 cells, these results strongly indicate a role of villus-like Caco-2 cells in HCO3--potentiated secretion. Thus, it appears that colonic Cl- and HCO3- potentiated secretion may be differentially mediated by different colonic cell types.
The observed difference between T84 and Caco-2 cells in their ISC responses to different concentrations of TMP suggested that colonic Cl- and HCO3- mediated secretions might be differentially elicited by the same agent depending on the concentrations used, probably due to differential expression of receptors, cotranspoters, exchangers and ion channels[10-13] leading to differential responses to TMP. This notion is supported by the observation that TMP at different concentrations could elicit different ISC responses from freshly isolated rat colon mucosa with distinct current characteristics. Interestingly, the colonic mucosal ISC response elicited by 1 mmol/L of TMP had characteristics similar to the TMP-induced response in T84 cells (e.g. sensitive to Cl- removal and bumetanide but insensitive to DIDS), indicating predominant Cl- secretion, whereas the colonic mucosal ISC response elicited by TMP at concentrations greater than 5 mmol/L resembled the response observed in Caco-2 cells (e.g., more sensitive to DIDS than to Cl- removal and bumetanide), indicating predominant HCO3--potentiated secretion at this concentration range. The fact that distinct TMP-induced ISC responses with characteristics similar to those observed in human colonic T84 and Caco-2 cells could be observed in intact rat colonic mucosa suggests that the secretory activities of T84 and Caco-2 cells may reflect the secretory properties of the colon, which may be similar in rats and humans. The present findings suggest that by having different sensitivities to stimulation in distinct colonic cell types, the colon in rats or humans, may be able to secrete either Cl--rich fluid or HCO3--rich fluid. In other words, colonic Cl-and HCO3--mediated secretions may be differentially regulated by the same physiological regulator depending on its local concentration. Possible candidates of physiological regulators may include prostaglandins, VIP and secretin, all of which are known to be present in the colon and able to increase intracellular cAMP[30-32]. In fact, secretin has been shown to stimulate Cl- secretion in the colon[33] and HCO3-secretion in Caco-2 cells[26]. It should be noted that TMP has been shown to be a cAMP-evoking agent having an action similar to PDE inhibitor in antiplatelet activity[15]. The further study is required to identify the signaling pathway in mediating TMP effects on colonic cells.
The present finding of predominant HCO3- -mediated secretion by villus-like Caco-2 cells is of physiological interest. Although a constant pH microclimate at the luminal surface has been considered to have a decisive influence on the reabsorption of Cl- and absorption of weak electrolytes such as short-chain fatty acids[34], the mechanisms involved in the maintenance and regulation of the pH microclimate are poorly understood. A recent study has demonstrated that HCO3- is the most important factor in maintaining a constant pH microclimate at the luminal surface[35]. However, the origin of HCO3- responsible for this was not demonstrated although a continuous secretion of HCO3- at the luminal surface of the colonic epithelium has been observed[36]. The present study suggested that the villus/surface-like epithelial cells were capable of secreting HCO3- upon stimulation, and thus, they are likely to contribute to the maintenance and regulation of the pH microclimate observed at the luminal surface of the colon. Being situated at the luminal surface, villus epithelial cells have the advantage over crypt cells in that they can sense the small variations in the pH microclimate at the luminal surface and secrete HCO3- directly adjusting the luminal microclimate. This suggests that the secretory role of villus/ surface epithelium is distinct from that of crypts which are mainly responsible for Cl- secretion on which colonic fluid secretion depends. The differential expression of NBC, the major basolateral transport mechanism for HCO3- entry[23], in the two human colonic cells lines also supports this contention.
The mechanism(s) by which Cl- and HCO3- exit through the apical membrane remains to be elucidated. The fact that both Cl- and HCO3- secretions could be completely blocked by DPC and glibenclamide, both of which are known to inhibit CFTR, suggest that a common mechanism may be involved. CFTR appears to be a possible candidate since its involvement in Cl-and HCO3- secretion has been demonstrated[5-7]. The investigation of detail mechanism involved in mediating the TMP-stimulated anion secretions is currently undertaken in our laboratory.
It should be noted that while TMP has been used clinically to treat cardiovascular disorders[14], no study has been reported, at the time of writing, on its effect on colonic secretion. The present results suggest that potential application of TMP as an alternative treatment of GI disorders may be further explored, considering its natural origin and differential effects on colonic Cl- and HCO3- secretion.