Published online Jun 15, 2004. doi: 10.3748/wjg.v10.i12.1740
Revised: December 15, 2003
Accepted: December 22, 2003
Published online: June 15, 2004
AIM: To study interactions between hepatitis B virus (HBV) and interferon-α in liver-derived cells.
METHODS: mRNAs were separately isolated from an HBV-transfected cell line (HepG22.2.15) and its parental cell line (HepG2) pre- and post-interferon-α (IFN-α) treatment at 6, 24 and 48 h, followed by hybridization with a cDNA microarray filter dotted with 14 000 human genes. After hybridization and scanning of the arrays, the data were analyzed using ArrayGauge software. The microarray data were further verified by Northern blot analysis.
RESULTS: Compared to HepG2 cells, 14 genes with known functions were down-regulated 3 to 12-magnitudes, while 7 genes were up-regulated 3-13 magnitudes in HepG22.2.15 cells prior to IFN-α treatment. After interferon-α treatment, the expression of four genes (vascular endothelial growth factor, tyrosine phosphate 1E, serine protein with IGF-binding motif and one gene of clathrin light chain) in HepG22.2.15 were up-regulated, while one gene encoding a GTP-binding protein, two genes of interferon-induced kinases and two proto-oncogenes were further down-regulated. Interestingly, under IFN-α treatment, a number of differentially regulated genes were new ESTs or genes with unknown functions.
CONCLUSION: The up-regulated genes in HepG22.2.15 cell line suggested that under IFN-α treatment, these repressed cellular genes in HBV infected hepatocytes could be partially restored, while the down-regulated genes were most likely the cellular genes which could not be restored under interferon treatment. These down-regulated genes identified by microarray analysis could serve as new targets for anti-HBV drug development or for novel therapies.
- Citation: Wang X, Yuan ZH, Zheng LJ, Yu F, Xiong W, Liu JX, Hu GX, Li Y. Gene expression profiles in an hepatitis B virus transfected hepatoblastoma cell line and differentially regulated gene expression by interferon-α. World J Gastroenterol 2004; 10(12): 1740-1745
- URL: https://www.wjgnet.com/1007-9327/full/v10/i12/1740.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i12.1740
Viral hepatitis B continues to be a significant global problem. It is estimated that there are approximately 350 million chronic hepatitis B virus (HBV) carriers world-wide. Furthermore, chronic hepatitis B patients are at high risk of developing liver cirrhosis and hepatocellular carcinoma with high mortality rates.
In the past two decades, interferon-α (IFN-α) has proven effective in the treatment of chronic hepatitis B patients. However, sustained responses were observed in only one-third of chronic hepatitis B patients[1-3]. In the normal physiological state, interferon-α is expressed at a low level and is induced to high levels by viral or bacterial infections and the exposure to double-stranded RNA[4]. In contrast to other types of viral infections, IFN-α was either non-detectable or present in extremely low levels in HBV chronically infected patients[5,6]. Additionally, decreased synthesis of 2’-5’ A synthetase, belonging to a family of enzymes induced by IFNs, has been reported in chronic hepatitis B patients[7,8]. To date, mechanisms underlying defective production of IFN or defective responses to IFN in chronic hepatitis B patients have not been fully elucidated.
Development of microarray technology has provided a powerful tool for study of the complicated biological process in cells which results in altered global gene expression. cDNA microarray has been used to analyze virus-host cell interactions in Human Immunodeficiency Virus (HIV), Human Cytomegalovirus (HCMV), Herpes Simplex Virus (HSV) and Influenza Virus infected cell cultures[9-13]. By comparing virus infected cell cultures with non-infected cell cultures, a number of differentially expressed genes were identified. For HBV, although several studies have been undertaken to clarify the effects of HBV infection on hepatocytes[14-16], the global effects of all HBV proteins or HBV replication on host cells, especially the interaction between HBV and interferon remain unclear. Therefore, in this study, cDNA microarray filters dotted with 14 000 human genes were used to analyze transcriptional changes between an HBV DNA-transfected cell line (HepG22.2.15)[17,18] and its parental cell line (HepG2) pre- and post- IFN treatment. Based on analysis of altered mRNA expression, several IFN-differentially regulated genes including new ESTs or genes with unknown functions were further investigated for their potential antiviral activity.
The HBV DNA transfected hepatoblastoma cell line HepG22.2.15, that has been confirmed to produce infectious virus[18,19], and its parental cell line HepG2 were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10 mL/L fetal calf serum and antibiotics. The cells were incubated at 37 °C in 50 mL/LCO2. Cell viability was estimated by the trypan blue dye exclusion method.
The cells were separately seeded into T-75 flasks at 40% confluence (4×104 cells/cm2) one day prior to the experiment. After overnight culture, two flasks of cells for each cell line were harvested before treatment with IFN-α for RNA preparation and were regarded as 0 h cells for IFN-α treatment. The other cells were exposed to 5×103 IU/mL of IFN-α (Calbiochem, San Diego, CA, USA) and harvested 6, 24 and 48 h after IFN-α treatment. The culture media were removed and stored at -70 °C. For each flask of cells, 5 mL of TRIzol Reagent (Gibco BRL) was added directly into the flask.
Total RNA was extracted using standard TRIzol RNA isolation protocol (Gibco BRL). The isolated total RNA was divided into two aliquots, one for RNA slot analysis and the other for mRNA preparation.
Generation of microarrays and microarray analysis were carried out by the Institute of Biochemistry and Cell Biology, CAS as described previously[20]. Human cDNA clones were derived from liver, hepatocarcinoma cell lines and hypothalamus-pituitary-adrenal libraries[21] or purchased from Research Genetics (Huntsville, AL, USA). A cDNA array was assembled with 14 000 cDNA clones representing the same number of independent cDNA clusters, of which 7 565 clusters were homologous to that in the UniGene Database. All cDNA fragments were amplified and verified by gel electrophoresis and sequencing. The average length of the cDNA fragments was -1 kb. PCR products were precipitated in isopropanol, redissolved in 10 μL of denaturing buffer (1.5 mol/L NaCl, 0.5 mol/L NaOH) and spotted onto 8×12 cm Hybond-N nylon membranes (Amersham Pharmacia, Buckinghamshire, UK) using an arrayer (BioRobotics, Cambridge, UK). Each spot carried -100 nL in volume and was 0.4 mm in diameter; each cDNA fragment was spotted as two different spots (double-offset). Lambda phage and pUC18 vector DNA were spotted as negative controls.
Eight housekeeping genes encoding ribosomal protein S9 (RPS9), β-actin (ACTB), glyceraldehydes-3-phosphate dehydrogenase, hypoxanthine phosphoribosyltransferase 1, Mr 23 000 highly basic protein (RPL13A), ubiquitin C, phospholipase A2, and ubiquitin thiolesterase (UCHL1) were each evenly distributed in 12 spots on each 8×12 cm array as an intramembrane control. Hybridization data were considered invalid if the intensity of the darkest spot exceeded 1.5 times that of the weakest spot among the 12 spots representing the same gene.
The poly (A)+ mRNA was isolated from the total RNA using a poly (dT) resin (Qiagen, Hilden, Germany). Approximately 1-2 μg of mRNA were labeled in a reverse transcription reaction in the presence of 200 μCi [α33P] deoxyadenosine 5’-triphosphate (DuPont NEN, Boston, MA, USA) using Moloney murine leukemia virus reverse transcriptase as recommended by the manufacturer (Promega Corp., Madison, WI, USA).
Prehybridization was carried out in 20 mL of prehybridization solution (6×SSC, 5 g/L SDS, 5×Denhardt’s and 100 μg/mL denatured salmon sperm DNA) at 68 °C for 3 h. Overnight hybridization with the 33P-labeled cDNA in 6 mL of hybridization solution (6×SSC, 5 g/L SDS, and 100 μg/mL denatured salmon sperm DNA) was followed by stringent washing (0.1×SSC, 5 g/L SDS, at 65 °C for 1 h). Membranes were exposed to Phosphor Screen overnight and scanned with an FLA-3 000A Plate/ Fluorescent Image Analyzer (Fuji Photo Film, Tokyo, Japan). Radioactive intensity of each spot was linearly digitalized to 65 536 gray-grade in a pixel size of 50 μm in the Image Reader and recorded using the Array Gauge software (Fuji Photo Film, Tokyo, Japan). After subtraction of background (3 ± 3) chosen from an area where no cDNA was spotted, genes with intensities > 10 were considered as positive signals to ensure that they were distinguished from background with statistical significance > 99.9%. Normalization among arrays was based on the sum of background-subtracted signals from all genes on the membrane[22].
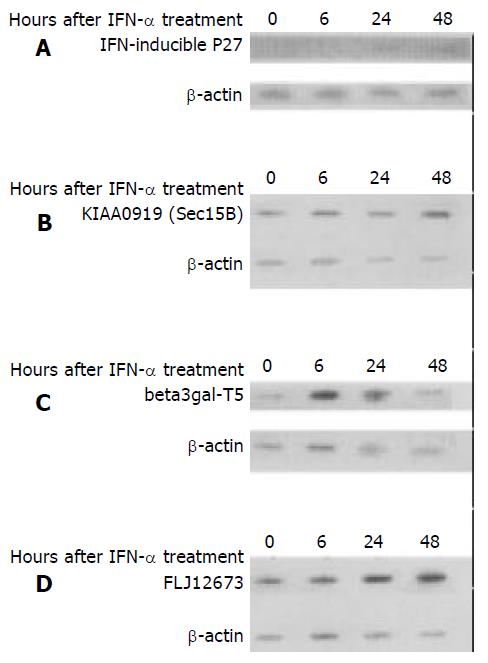
Total cellular RNA, 10 μg/sample was mixed with 7 μL formaldehyde, 20 μL formamide and 2 μL 20×SSC (1×SSC = 0.15 mol/L NaCl plus 0.015 mol/L sodium citrate). After being denatured at 65 °C for 15 min and immediately cooled in an ice bath, the RNA sample was applied to the nylon membrane (Boehringer Mannheim, Germany) with Minifold I (Schleicher & Schuell). After fixation at 120 °C for 30 min, the membrane was pre-hybridized in 20 mL of prehybridization solution (6×SSC, 5 g/L SDS, 5×Denhardt’s and 100 μg/mL denatured salmon sperm DNA) at 42 °C for 6 h, and followed by hybridization with 20 mL hybridization solution (6×SSC, 5 g/L SDS, 5×Denhardt’s and 100 μg/mL denatured salmon sperm DNA) including [α-32P]dCTP labeled cDNA probes (Random labeling kit, Boehringer Mannheim, Germany) at 42 °C for 16 h. The cDNA probes represented the unique sequence of IFN related P27, KIAA0919, beta3gal-T5 and FLJ12673. After stringent membrane washing process (0.1×SSC, 5 g/L SDS at 68 °C for 30 min, thrice), autoradiographs were exposed to X-ray film (Fijifilm, Japan) overnight at -70 °C. Quantitative analysis was undertaken by scanning the intensity of the membranes. In order to normalize the total RNA quantity in each blot, an [α32-P]dCTP labeled β-actin probe was hybridized to the membrane again after the first probe was removed. The change of regulated gene expression between different cell samples could be detected by comparing the intensity ratio of specific gene and β-actin.
Similar to previous study, results from the reproducible analysis confirmed that the replicated experiments were in concordance with an R2 (square of Pearson correlation coefficiency, measuring similarity in gene expression pattern) of 0.97-0.98 on scatterplot[20].
To confirm the altered expression level, 4 genes (interferon related genes P27, KIAA0919, β-3-gal-T5 gene and FLJ12673 human cDNA clone) were selected and tested by RNA slot analysis (Figure 1). The ratios of the signal intensity of specific genes and β-actin and their comparison with microarray result are listed in Table 1. It is observed that the results of RNA slot analysis are aligned with those from the microarray analyses (Figure 1 and Table 1).
| Accession | X67325 | AB023136 | AA622328 | NM_018300 | ||||
| ID | Ratio of arrays | Ratio of slot | Ratio of arrays | Ratio of slot | Ratio of arrays | Ratio of slot | Ratio of arrays | Ratio of slot |
| 6 h/0 h | 3.6 | Undetectable | 3.4 | 1.3 | 2.0 | 0.5 | 1.1 | 2.9 |
| 24 h/0 h | 5.9 | > 31 | 7.2 | 3.5 | 3.9 | 1.6 | 4.9 | 3.4 |
| 48 h/0 h | 6.1 | > 33 | 8.4 | 6.2 | 3.8 | 5.7 | 1.0 | 1.3 |
| Description | IFN-inducible P27 | Sec15B(vesicle traffic protein) | beta3gal-T5 gene | cDNA FLJ12673 | ||||
To study the basic differentiated expressed genes between HepG2 2.2.15 cell line and its parental cell line HepG2, systematic comparison of gene expression profiles from both cell lines before treatment with IFN-α was done at 0 h. Totally 89 genes were either up- or down-regulated three-fold (Table 2). In these 89 genes, 22 genes have defined functional activities (Table 3) while 67 genes were new ESTs with unknown functions. Fifteen of the 22 known genes were down-regulated by three- to twelve-fold, while 7 genes were up-regulated by three- to thirteen-fold. 4 genes could be catalogued in four classes, such as cell cycle (Y13467), ligands and receptors (AF022375), phosphatase (U12128), and transcription factors (D87258).
| Ratio | Gene differences in HepG22.2.15 | Gene differences in HepG2 | Gene differences in HepG22.2.15/HepG2 | |||||||
| 6 h/0 h | 24 h/0 h | 48 h/0 h | 6 h/0 h | 24 h/0 h | 48 h/0 h | 0 h | 6 h | 24 h | 48 h | |
| 3 | 34 | 48 | 45 | 83 | 103 | 48 | 89 | 40 | 33 | 36 |
| 5 | 10 | 18 | 12 | 37 | 37 | 23 | 27 | 12 | 8 | 11 |
| Accession ID | HepG2 | HepG22.2.15 | Ratio | Description | Unigene |
| Cell cycle | |||||
| Y13467 | 101.95 | 29.54 | -3.45 | RB18A | Hs.15589 |
| Ligands and receptors | |||||
| AF022375 | 16.17 | 5.19 | -3.12 | vascular endothelial growth factor | Hs.73793 |
| Phosphatase | |||||
| U12128 | 152.03 | 27.26 | -5.58 | tyrosine phosphatase 1E (PTP1E) | Hs.211595 |
| Transcription factors | |||||
| D87258 | 156.76 | 37.78 | -4.15 | serin protease with IGF-binding motif | Hs.75111 |
| Miscellaneous | |||||
| X69962 | 242.93 | 73.77 | -3.29 | FMR-1 | Hs.89764 |
| X56597 | 1.64 | 21.98 | 13.40 | humFib mRNA for fibrillarin | Hs.99853 |
| U76713 | 403.90 | 123.59 | -3.27 | apobec-1 binding protein 1 | Hs.81361 |
| U50410 | 189.94 | 27.03 | -7.03 | heparan sulphate proteoglycan (OCI5) | Hs.119651 |
| U23070 | 5.35 | 16.82 | 3.14 | putative transmembrane protein (nma) | Hs.78776 |
| S68805 | 4.12 | 12.74 | 3.09 | L-arginine:glycine amidinotransferase | Hs.75335 |
| M22430 | 13.63 | 50.17 | 3.68 | RASF-A PLA2 | Hs.76422 |
| M16961 | 221.65 | 61.50 | -3.60 | alpha-2-HS-glycoprotein alpha and beta chain | Hs.75430 |
| M13928 | 21.15 | 163.32 | 7.72 | delta-aminolevulinate dehydratase | Hs.1227 |
| L40157 | 14.58 | 92.98 | 6.38 | endosome-associated protein (EEA1) | Hs.2864 |
| L24123 | 1 309.05 | 430.92 | -3.04 | NRF1 protein (NRF1) | Hs.83469 |
| D00096 | 7.61 | 31.28 | 4.11 | Prealbumin | Hs.194366 |
| AJ006835 | 150.29 | 49.21 | -3.05 | U17 small nucleolar RNA host gene | Hs.196769 |
| AF073308 | 237.11 | 78.45 | -3.02 | nonsyndromic hearing impairment protein (DFNA5) | Hs.13530 |
| AF050127 | 823.97 | 71.21 | -11.57 | hypoxia inducible factor (aHIF) | Hs.197540 |
| AC002045 | 237.87 | 67.71 | -3.51 | Chromosome 16 BAC clone CIT987SK-A-589H1 | Hs.253182 |
| AB027013 | 15.62 | 2.31 | -6.76 | Nucleosome Assembly Protein 1-like 2 | Hs.66180 |
| AB023136 | 544.25 | 44.18 | -12.32 | KIAA0919 | Hs.44175 |
Six hours after treatment with interferon-α, significant changes in mRNA expression were observed in both HepG2 and HepG22.2.15 cells, with 83 and 34 genes up- or down-regulated by at least three-fold (Table 2). A moderate increase in changes of cellular gene expression was detected at 24 h, at which time, 103 and 48 genes were up- or down-regulated respectively by at least three-fold (Table 2). Approximately half of these genes could be classified in functional categories, including interferon responsive proteins, signal transducing genes such as kinase, phosphatase and ligand as well as receptors (Table 4). It is interesting to note that the number of differentially expressed cellular genes in HepG2 and HepG22.2.15 cell lines could be decreased by interferon-α treatment from 89 to 40, 33 and 36 genes at 6, 24, 48 h post treatment (Table 2). The differentially regulated gene expressed profile in HepG2 2.2.15 and HepG2 cell lines after IFN-α treatment are available at http://www.mvlab-fudan.cn/microarray databases.
| Catalogue | 3-fold regulated gene number in HepG22.2.15 | 3-fold regulated gene number in HepG2 | ||||
| 6 h/0 h | 24 h/0 h | 48 h/0 h | 6 h/0 h | 24 h/0 h | 48 h/0 h | |
| Cell cycle | - | 1 | 1 | - | - | - |
| Coagulation | - | 1 | 1 | - | 1 | - |
| Cytokine/receptors | 1 | 2 | 2 | 1 | 1 | - |
| Extracellular matrix and cell adhesion | - | - | 1 | - | 1 | - |
| GTP binding proteins and signal transduction | 2 | - | 1 | - | 1 | 1 |
| Interferon | 8 | 6 | 3 | 6 | 5 | 5 |
| Kinase and phosphatase | - | - | 1 | - | 2 | - |
| Ligands and receptors | - | 1 | 2 | - | - | - |
| Mitochondrial | - | 1 | 1 | - | 2 | - |
| Phosphatase and phosphodiesterase | 2 | 2 | 2 | 1 | 2 | 1 |
| Protooncogenes | - | 1 | - | 1 | - | - |
| Transcription factors | - | - | - | 1 | 1 | 1 |
| Viral and clathrin receptors | - | 1 | 1 | - | 1 | - |
| Miscellaneous | 7 | 8 | 8 | 14 | 18 | 13 |
| New ESTs | 14 | 24 | 21 | 59 | 68 | 27 |
| Total | 34 | 48 | 45 | 83 | 103 | 48 |
Twenty three selected genes, belonging catalogues of interferon induced proteins kinase/phosphatase, ligands and receptors, phosphatase, protein degradation, viral and clathrin receptors, protooncogenes and transcription factors respectively, were further compared between HepG22.2.15 and its parental cell line HepG2 to study the effect of interferon-α treatment on HBV and cellular gene expressions (Table 5). From this table, it is noted that most of these selected genes with known functions have two- three-fold expression regulated in both HepG2 cell line and HepG22.2.15 cell post interferon treatment. However, five genes (U02680, U07358, U52969, U50529 and U70321), were up-regulated only in HepG2 cell line, while down-regulated in HepG22.2.15 cells.
| Accession ID | HepG22.2.15 cell lines | HepG2 cell lines | Description | ||||
| 6 h/0 h | 24 h/0 h | 48 h/0 h | 6 h/0 h | 24 h/0 h | 48 h/0 h | ||
| Interferon | |||||||
| X57352 | 3.8 | 3.1 | 2.5 | 6.5 | 8.1 | 6.4 | 1-8U gene from interferon-inducible gene family |
| X57351 | 3.4 | 2.2 | 2.2 | 4.3 | 4.4 | 4.4 | 1-8U gene from interferon-inducible gene family |
| M87503 | 2.3 | 1.7 | 1.7 | 3.2 | 2.4 | 2.6 | IFN-responsive transcription factor subunit |
| M33882 | 2.4 | 1.6 | 1.5 | 2.5 | 2.1 | 1.6 | p78 protein |
| M11810 | 9.1 | 5.7 | 4.4 | 8.4 | 7.7 | 3.9 | (2’-5’) oligo A synthetase E gene |
| L07633 | 2.0 | 1.3 | 1.5 | 2.0 | 2.7 | 1.3 | (clone 1950.2) interferon-gamma IEF SSP 5111 |
| J04164 | 6.7 | 5.0 | 2.9 | 6.4 | 7.0 | 6.5 | Interferon-inducible protein 9-27 |
| D28137 | 3.0 | 3.3 | 2.8 | 2.7 | 2.6 | 1.8 | BST-2 |
| AJ225089 | 3.9 | 1.3 | 1.1 | 1.9 | 2.1 | 1.2 | 2’-5’ oligoadenylate synthetase 59 kDa isoform |
| Kinase/Phosphate | |||||||
| U07358 | -1.3 | -1.3 | -2.5 | 1.1 | 2.4 | 1.4 | Protein kinase (zpk) |
| U02680 | -1.1 | 1.4 | -2.5 | 1.5 | 7.1 | 1.6 | Protein tyrosine kinase |
| AF032437 | -1.1 | -2.5 | -1.7 | -1.3 | -3.3 | -2.0 | Mitogen activated protein kinase activated protein kinase |
| Ligands and receptors | |||||||
| D86096 | 2.3 | 4.6 | 3.5 | 1.9 | 1.8 | 2.3 | Prostaglandin E receptor EP3 subtype |
| AF022375 | 1.8 | 1.8 | 3.2 | -1.2 | -1.1 | 1.1 | Vascular endothelial growth factor |
| Phosphatase | |||||||
| U12128 | 3.0 | 4.8 | 4.7 | -1.2 | -1.1 | -1.2 | Protein tyrosine phosphatase 1E (PTP1E) |
| Protein degradation | |||||||
| Z14977 | 2.2 | 1.2 | 1.4 | 2.7 | 1.3 | 1.6 | Major histocompatibility complex encoded |
| proteasome subunit LMP2 | |||||||
| AF061736 | 2.6 | 1.5 | 1.4 | 2.3 | 2.3 | 2.0 | Ubiquitin-conjugating enzyme RIG-B |
| Protooncogenes | |||||||
| U52969 | 1.1 | -2.0 | -1.3 | 2.2 | 1.6 | 2.1 | PEP19 (PCP4) |
| U50529 | -2.5 | -5.0 | -2.5 | 1.7 | 1.5 | 2.2 | BRCA2 region, mRNA sequence CG016 |
| Transcription factors | |||||||
| D87258 | 1.6 | 2.3 | 2.5 | 1.0 | -1.1 | -1.5 | Serin protease with IGF-binding motif |
| Viral and clathrin receptors | |||||||
| X81636 | 1.1 | 5.5 | 5.2 | -1.1 | 6.4 | -1.7 | Clathrin light chain a |
| U70321 | -1.3 | -2.0 | 1.1 | 2.0 | 1.2 | 1.6 | Herpesvirus entry mediator |
| AF079221 | 1.0 | 1.2 | 2.9 | 1.7 | 2.2 | 1.9 | BCL2/adenovirus E1B 19kDa-interacting protein 3 a |
DNA microarray is an important and powerful tool for surveying changes in cellular gene expression on a large scale. Several groups have used this approach to analyze virus-host cell interactions in HBV infection. Rho et al[15] constructed a HepG2 cell line stably expressing HBx (HepG2-HBx), and performed cDNA microarray analysis on 588 cellular cDNAs to study the effect of HBx on the transcriptional regulation in the human liver cell. To gain more information on how HBV proteins regulate host cellular gene expression, Otsuka et al[16] examined the differences in the gene expression profiles of HepG22.2.15 and HepG2 cells, using an in-house cDNA microarray dotted with 2 304 cellular genes. To identify hepatocellular genes whose transcriptional regulation is tightly linked with IFN-γ- and IFN-α/β-mediated inhibition of HBV replication, Chisari et al[23] used DNA microarrays and a high-throughput cDNA differential display method (total gene expression analysis (TOGA) to examine the gene expression profiles of HBV transgenic mouse livers before and after the intrahepatic induction of IFN-α/β and IFN-γ. To further understand molecular mechanisms underlying HBV pathogenesis and identify novel cellular genes as new targets for anti-HBV drug development or for novel therapies, we used cDNA microarray filters dotted with 14 000 human genes to analyze transcriptional changes between an HBV DNA-transfected cell line (HepG2 2.2.15) and its parental cell line (HepG2) pre- and post-IFN treatment. The HepG22.2.15 cell line, that has four copies of HBV genome integrated in the cellular chromosome and can persistently secrete HBV virions, was used for HBV infected liver-derived cells[17,18]. The parental hepatoblastoma cell line, HepG2, from which HepG22.2.15 cell line was generated, was used as the uninfected cell control. In this study, the differentially expressed cellular genes detected between these two cell lines could be due to the integration of HBV DNA, the replication of HBV or the expression of HBV proteins. In chronic HBV infections, the virus usually persists in hepatocytes similar to the status of HBV in HepG22.2.15 cell line; the differentially expressed cellular genes in HepG22.2.15 cell line could reveal similar changes in the hepatocytes of HBV chronically infected patients.
Prior to interferon-α treatment, compared to HepG2 cells, 15 known genes were more than three-fold down-regulated, while 7 genes were more than 3-fold up-regulated (Table 3) in HepG2 2.2.15 cells. To concentrate on the down-regulated genes, 4 genes belonging to the categories of cell cycle, ligands and receptors, phosphatase and transcription factors were down-regulated more than three-fold in HepG2 2.2.15 cells (Table 3). In a microarray study on HCMV-host cell interactions compared to non-infected human fibroblasts, down-regulated genes belonging to these categories were also reported in HCMV infected cells[10]. Though identical down-regulated genes from these categories were not found in the HCMV infected and HBV transfected cells, changes in the expression of cellular genes in these categories could reflect the common adverse effects of virus infections on host cells. In future studies, when more microarray analyses on virus-host interactions will be performed, it may be possible to identify significant changes of cellular gene expression due to HBV-hepatocyte interactions.
The effects of IFN-α on the global gene expression of HepG2 and HepG22.2.15 cells revealed interesting differences (data available at http://www.mvlab-fudan.cn/microarray databases). In HepG2 cells, compared with the expression of cellular genes at 0 h, no known gene was three-fold down-regulated 48 hr after IFN-α treatment. In contrast, 21 genes were up-regulated three-fold or more, and among these, five were IFN-induced genes, one belonging to the GTP-binding protein and signal transduction category, and one belonging to the phosphatase and phosphodiesterase category associated with the IFN-induced cascade. In HepG2 2.2.15 cells, after being treated with IFN-α for 48 h, eight genes remained down-regulated three-fold or more. In comparison to the known functional genes, one gene was predicted as encoding the regulatory G protein beta subunit, one was predicted as encoding an antigen of B cell differentiation, one encoding the thymosin beta 4Y isoform, one encoding a LDL-receptor related protein, which was also reported as encoding a human IFN-γ receptor, and one was an exon encoding a protein of the phosphatase and phosphodiesterase category, and the other 3 genes belonged to coagulation, mitochondrial and miscellaneous categories, respectively. These genes remained down-regulated and thus seemed refractory to the treatment of IFN-α. Since 2 of these genes are associated with thymosin and IFN-γ, microarray studies of HepG22.2.15 cells treated with IFN-γ and/or cytokines may be able to elucidate whether these defects in cellular functions could be reversed. In HepG22.2.15 cells, a number of IFN induced genes were up-regulated post IFN-α treatment; among these, genes p27 and 2’-5’A synthetase E were the top two up-regulated genes, being 6.1 and 9.1 respectively. Compared to the increases in these two genes up-regulated in HepG2 (being 7.1 and 8.4 respectively), IFN-α was as effective in inducing these two genes in HBV infected cells as that in the uninfected hepatocytes.
To analyze whether there were HBV specific down-regulated cellular genes, which could be restored by IFN-α treatment, 23 matched genes from HepG2 and HepG22.2.15 cell lines were compared post IFN-α treatment. As shown in Table 5, the three genes which were found down-regulated in HepG22.2.15 prior to IFN-α treatment (Table 3), namely described as encoding vascular endothelial growth factor, tyrosine phosphatase 1E, serine protease with IGF-binding motif were all up-regulated by IFN-α treatment. In addition, a gene described as coding for clathrin light chain, which belongs to the viral and clathrin receptor category was also up-regulated 5.2 times. However, even after IFN-α treatment, the down-regulated protein kinase zpk and protein tyrosine kinase genes could not be restored (Table 5), which suggested a possible defective link in the IFN-α induced cascade. Measures for restoring the functions of the kinases could help to improve the therapeutic efficacy of IFN-α. The clinical implications of non-restorable down-regulated protooncogenes in HepG2 2.2.15 cell lines merit further study.
Analysis of gene expression profiles in HBV-hepatocyte interaction by microarray is an interesting field. The information gathered in this study, though preliminary in scope, provides an important basis for further study. The differentially expressed genes detected in this study should be further evaluated by cell transfection or by in vivo studies and should provide new insight into the molecular mechanisms of HBV infection. In addition, there were approximately 200 unknown genes or ESTs being up- or down-regulated in HepG22.2.15 cells. Preliminary transiently co-transfected functional assay has already shown that some of cellular genes significantly regulated by HBV infection and IFN are associated with the inhibition of HBV gene replication and expression (data not shown). It is predicted that other IFN-inducible proteins could be discovered and new mechanisms of this cytokine may be revealed.
This work was supported by the Chinese State Basic Science Foundation (No.1999054105) and Med-X Foundation of Fudan University. Xun Wang and Wei Xiong are Ph.D. students supported by the Chinese Ministry of Education.
Edited by Xu FM and Wang XL
| 1. | Hoofnagle JH. Alpha-interferon therapy of chronic hepatitis B. Current status and recommendations. J Hepatol. 1990;11:S100-S107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Korenman J, Baker B, Waggoner J, Everhart JE, Di Bisceglie AM, Hoofnagle JH. Long-term remission of chronic hepatitis B after alpha-interferon therapy. Ann Intern Med. 1991;114:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 267] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 3. | Caselmann WH, Eisenburg J, Hofschneider PH, Koshy R. Beta- and gamma-interferon in chronic active hepatitis B. A pilot trial of short-term combination therapy. Gastroenterology. 1989;96:449-455. [PubMed] |
| 4. | Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778-809, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2029] [Cited by in RCA: 2000] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 5. | Wen YM, Qian LS, Lou HZ, Gao JQ, Wu XH, Yang PZ. Stud-ies on production of interferon I by peripheral blood mono-nuclear cells in chronic hepatitis B patients. Chin Med J. 1980;60:239-241. |
| 6. | Wen YM, Luo HZ, Qian LS, Duan SC, Zhu QR, Wu XH. Serial study on production of interferon by leucocytes in viral hepati-tis B. Chin J Microb Immunol. 1981;1:342-345. |
| 7. | Wen YM, Yu CZ, Huang YX, Wu XH, Zheng HD, Liu XY. Studies on 2'-5' oligo-isoadenylate synthetase and virus repli-cation in hepatitis B. Chin J Inf Dis. 1985;3:106-109. |
| 8. | Fujisawa K, Yamazaki K, Kawase H, Kitahara T, Kimura K, Ogura K, Kameda H. Interferon therapy for chronic viral hepa-titis and the use of peripheral lymphocytic 2'-5'-oligoadenylate synthetase. Viral hepatitis and liver disease. New York: Alan R Liss Inc 1988; 834-839. |
| 9. | Geiss GK, Bumgarner RE, An MC, Agy MB, van 't Wout AB, Hammersmark E, Carter VS, Upchurch D, Mullins JI, Katze MG. Large-scale monitoring of host cell gene expression during HIV-1 infection using cDNA microarrays. Virology. 2000;266:8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 193] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Zhu H, Cong JP, Mamtora G, Gingeras T, Shenk T. Cellular gene expression altered by human cytomegalovirus: global monitoring with oligonucleotide arrays. Proc Natl Acad Sci USA. 1998;95:14470-14475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 334] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 11. | Browne EP, Wing B, Coleman D, Shenk T. Altered cellular mRNA levels in human cytomegalovirus-infected fibroblasts: viral block to the accumulation of antiviral mRNAs. J Virol. 2001;75:12319-12330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 225] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 12. | Stingley SW, Ramirez JJ, Aguilar SA, Simmen K, Sandri-Goldin RM, Ghazal P, Wagner EK. Global analysis of herpes simplex virus type 1 transcription using an oligonucleotide-based DNA microarray. J Virol. 2000;74:9916-9927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 120] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Geiss GK, An MC, Bumgarner RE, Hammersmark E, Cunningham D, Katze MG. Global impact of influenza virus on cellular pathways is mediated by both replication-dependent and -independent events. J Virol. 2001;75:4321-4331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 101] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Lara-Pezzi E, Majano PL, Gómez-Gonzalo M, García-Monzón C, Moreno-Otero R, Levrero M, López-Cabrera M. The hepatitis B virus X protein up-regulates tumor necrosis factor α gene expression in hepatocytes. Hepatology. 1998;28:1013-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 99] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Han J, Yoo HY, Choi BH, Rho HM. Selective transcriptional regulations in the human liver cell by hepatitis B viral X protein. Biochem Biophys Res Commun. 2000;272:525-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Otsuka M, Aizaki H, Kato N, Suzuki T, Miyamura T, Omata M, Seki N. Differential cellular gene expression induced by hepatitis B and C viruses. Biochem Biophys Res Commun. 2003;300:443-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Knowles BB, Howe CC, Aden DP. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980;209:497-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1473] [Cited by in RCA: 1637] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 18. | Sells MA, Chen ML, Acs G. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc Natl Acad Sci USA. 1987;84:1005-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 939] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 19. | Gerber MA, Sells MA, Chen ML, Thung SN, Tabibzadeh SS, Hood A, Acs G. Morphologic, immunohistochemical, and ultrastructural studies of the production of hepatitis B virus in vitro. Lab Invest. 1988;59:173-180. [PubMed] |
| 20. | Xu L, Hui L, Wang S, Gong J, Jin Y, Wang Y, Ji Y, Wu X, Han Z, Hu G. Expression profiling suggested a regulatory role of liver-enriched transcription factors in human hepatocellular carcinoma. Cancer Res. 2001;61:3176-3181. [PubMed] |
| 21. | Hu RM, Han ZG, Song HD, Peng YD, Huang QH, Ren SX, Gu YJ, Huang CH, Li YB, Jiang CL. Gene expression profiling in the human hypothalamus-pituitary-adrenal axis and full-length cDNA cloning. Proc Natl Acad Sci USA. 2000;97:9543-9548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Rhee CH, Hess K, Jabbur J, Ruiz M, Yang Y, Chen S, Chenchik A, Fuller GN, Zhang W. cDNA expression array reveals heterogeneous gene expression profiles in three glioblastoma cell lines. Oncogene. 1999;18:2711-2717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Wieland SF, Vega RG, Müller R, Evans CF, Hilbush B, Guidotti LG, Sutcliffe JG, Schultz PG, Chisari FV. Searching for interferon-induced genes that inhibit hepatitis B virus replication in transgenic mouse hepatocytes. J Virol. 2003;77:1227-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |