Published online Jun 15, 2004. doi: 10.3748/wjg.v10.i12.1709
Revised: October 5, 2003
Accepted: October 12, 2003
Published online: June 15, 2004
The development of T helper 1 versus T helper 2 cells is a major branch point in the immune response and is an important determinant of the body’s response to an infectious pathogen, leading to protection of the host or dissemination of the disease. Resent studies have shown that there exist macrophage activation states in parallel to the T helper cell type 1/2 paradigm, and the T helper 1 development process is governed to a great degree by cytokine IL-12 provided mainly by antigen presenting cells such as macrophages and dendritic cells. A model in patients with hepatitis is proposed that links the pathogen, macrophage activation and T helper cell polarization.
- Citation: Sun QL, Ran W. Review of cytokine profiles in patients with hepatitis. World J Gastroenterol 2004; 10(12): 1709-1715
- URL: https://www.wjgnet.com/1007-9327/full/v10/i12/1709.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i12.1709
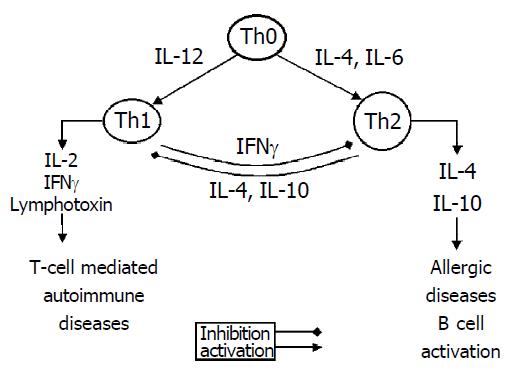
The immune response that occurs during infectious disease is characterized by plasticity in both its nature and its magnitude. This feature provides an important advantage that permits the immune system to tailor its defense strategy to particular groups of infectious pathogens. Interactions between CD4+T helper (Th) lymphocytes and antigen-presenting-macrophage cells in liver shape and amplify the subsequent immune response. The Th precursors differentiate into 2 subsets of effector Th cells with different functions[1,2]. Th1 cells produce IFN-γ, IL-2 and Th2 cells produce IL-4, IL-10 and IL-13. The ensuing Th1- and Th2-type immune responses include both potent humoral and cell-mediated components. Th1-derived IFN-γ suppresses Th2 response and Th2-derived IL-10 inhibits the development of Th1 populations[3]. In addition, the effector cells and antibody isotypes involved are quite distinct[4]. Th1 cells are responsible for activation of macrophages to a microbicidal state, induction of IgG2 a Abs that mediate phagocytosis, and support of CD8+ antiviral effector T cells. By contrast, Th2 cells stimulate the growth and differentiation of mast cells and eosinophils, as well as the production of Ab isotypes, including IgE and IgG4, which can mediate the activation of these cells. IL-12, a cytokine elaborated mainly by macrophages/monocytes, induces the maturation of Th1 cells. An imbalance of Th1 and Th2 appears to be important in the pathogenesis of chronic viral and nonviral infections in humans, such as infection with human immunodeficiency virus, leprosy, leishmaniasis[5-7],and viral hepatitis[8].
Recent studies have shown macrophage activation states in parallel to the Th cell type 1/2 paradigm. IFN-γ has long been known as the classical macrophage activating factor inducing cytokine secretion by macrophages to support Th1-driven immune responses. IL-4 which was historically regarded as macrophage deactivators is now thought to induce alternative immunological activation of macrophages[9], in that it enhances of the capacity of macrophages for endocytosis and antigen presentation by the induction of mannose receptor expression[10]. In normal immunological process, classical and alternative macrophage activation maintains the balance of macrophage.
Chronic hepatitis is characterized by incomplete clearance of virus and damage hepatocytes. Since hepatitis B virus (HBV) is known to have no cytopathic effect on the infected hepatocytes, cell-mediated immunity is thought to play an important role in the pathogenesis of hepatocellular damage and HBV clearance[11]. Although immune evading mechanisms used by HBV are largely unknown, defects in T cell response have been suspected as a major factor involved in the pathogenesis of chronic hepatitis[11-15]. Different from acute self-limited hepatitis in which protective immunity develops after elimination of HBV, immune response fails to remove HBV-infected hepatocytes in chronic hepatitis B. Th1 type pattern of secreted immunity can be considered as an appropriate response of the immune system to inhibit viral replication and HBV eradication. Mechanisms by which IFN-γ favors the elimination of HBV may include enhancement of cytotoxic T lymphocyte (CTL) activation, direct anti-viral activity, increased expression of major histcompatibility complex class I molecules on infected cells, and activation of macrophages[16-18]. It has been shown in a transgenic mouse model that adoptive transfer of CTLs producing IFN-γ could inhibit HBV replication without cytolysis[19].
Studies have shown[18] that predominant Th1 (IFN-γ) cytokine profile of hepatitis B core Ag (HbcAg)-specific and hepatitis B surface Ag (HbsAg)-reactive T cells is associated with acute self-limited hepatitis B. In most patients with acute hepatitis, CTL responses to epitopes of HBsAg, while there are no such responses in patients with chronic hepatitis[20]. Thus, Th1 might be insufficient for complete removal of HBV in chronic hepatitis and positively correlated with hepatic inflammatory activity.
Meanwhile, Barnaba et al[21] cloned CD4+ HBV envelope antigen (HbeAg)-reactive T cells and showed signs of cytotoxicity only when they produced IFN-γ. This is in agreement with findings that production of IFN-γ[22] by peripheral blood mononuclear cells (PBMCs) upon HBsAg stimulation was associated with higher level of hepatocyte damage. The dissociation between the mechanisms responsible for the immune-mediated hepatocytolysis, and for vural clearance in HBV infection was proposed[23]. It has been demonstrated that the interaction between Ag-specific CTL and target hepatocytes results in spotty necrosis which is limited to a very few hepatocytes[24]. Instead, the antiviral effect is mediated by IFN-γ, IL-2, and TNF-α released by HBV-specific CTLs or by antigen nonspecific macrophages, and these cytokines profoundly suppress HBV gene expression in infected hepatocytes by noncytolytic mechanisms[25] through eliminating HBV nucleocapsid particles and destabiling the viral RNA[26]. Thus, after recognition of HBV antigens on the surface of infected hepatocytes, CTLs perform two distinct functions; they kill a small fraction of infected hepatocytes and secrete IFN-γ and TNF-α, which exert antiviral effects without destruction of hepatocytes. Effective clearance of duck HBV and woodchuck hepatitis virus has also been shown to occur without massive hepatocellular necrosis[27].
In addition, a cytokine balance favoring Th2 type cytokine production such as IL-4 and IL-10 has been associated with progressive virus infections. Co-activation of Th3 cells with Th2 cells can negatively regulate immune responses and may be associated with the immune tolerant state of chronic HBV infection[28]. The shift from Th1 to Th2 or Th0 profiles was observed in acquired immune deficiency syndrome[29]. Result also indicates the existence of a Th2 type response to HBsAg in chronic hepatitis B patients with more sever liver damage[30]. Th2 cells may be associated with the persistence of HBV infection[31]. Probably the virus can mutate effectively and evade T-cell immune defense mechanisms. Persistent infection upsets the balance between immunostimulatory and inhibitory cytokines which can prolong inflammation and lead to necrosis, fibrosis, and chronic liver disease[32].
In chronic hepatitis C virus (HCV) infection, Fan et al[33] observed that the elevated levels of Th2 cytokines were greater than Th1 cytokines measured by ELISA. This result is in agreement with Kobayashi et al[34] who showed a significant increase in number of IL-4-producing Th2 cells and a significant decrease in number of IFN-γ-producing Th1 cells (PBMC stimulated by anti-CD3 antibody). They[34] suggested that, theoretically, stimulation with anti-CD3 antibody results in the expansion of T lymphocytes, whereas stimulation with HCV core protein results in the expansion of T lymphocytes responsive to HCV core antigen. The stimulation of PBMC with anti-CD3 antibody could reflect the patient’s real situation better than that with HCV core protein. In addition, they measured the IFN-γ after the depletion of CD8+ T cells - one of the major sources of IFN-γ production. This could explain that the increased production of IFN-γ (Th1) shown in others studies were due to IFN-γ secretion by CD8+T lymphocytes.
However, Iwata et al[35] found that in patients with chronic hepatitis C there was the increasing production of IFN-γ (Th1) by PBMC after stimulation with HCV core protein. Bergamini et al[36] obtained the similar result that the percentage of Th1 cells was significantly increased in CD4+, CD8+, ‘naive’-CD45RA+ and ‘memory’-CD45RO+ T-cell subsets (PBMC by mitogen-stimulation) from patients versus controls, and Sobue1 et al[37] also found that a shift to Th1 cytokine profile correlated with the progress of liver damage, which could be related to the higher proportion of CD4+T cells. Although in chronic hepatitis C infection, the levels of mononuclear cells derived from peripheral blood reflected the level of mononuclear cells derived from liver, the profile of Th1/Th2 in liver tissue and in peripheral blood was different[37]. In liver tissue, a predominance of Th1-type cytokines was seen in CD4+T cells[38], while a predominance of Th1 type cytokines was observed in CD8+ cells in peripheral blood. Thus, the elevated cytokine production may also have been caused by a higher proportion of CD4+T cells in the liver tissue of patients with chronic HCV infection compared with that in healthy controls, and intrahepatic CD4+T cells may be more important than CD8+T cells in the pathogenesis of liver damage in chronic hepatitis C infection. In addition, the percentage of CD4+T cells in liver correlated with the histological activity of hepatitis[38,39]. These results may indicate a preferential compartmentalization of Th1 cytokine-producing CD4+T cells in the liver[40], suggesting that some liver-derived CD4+T cells had a direct cytotoxic effect[41,21]. Meanwhile cytokines produced may inhibit viral replication, such as IFN-γ and TNF-α[26].
The probable mechanism of Th1 predominance in HCV infection is that in order to eliminate HCV and inhibit viral replication, the compartmentalized CD4+T cells may shift to a Th1 profile and induce nonspecific immune responses to activate nonspecific immune cells and effector molecules, resulting in liver cell damage. Nevertheless, further studies are needed to investigate the significance of CD4+T cells in liver tissue.
But the different result was observed regarding Th1 or Th2 predominance in patients with chronic hepatitis C, which was probably due to the administration of PBMC and the measurement for Th1 cytokines from CD4+T, or both CD4+ and CD8+ T cells. It is also possible that the HCV-related antigen influences T helper cells to produce a different cytokine profile from that in healthy subjects. For example, in transgenic mice with HBeAg, the T cell response against peptide 120-31 of HBeAg was predominantly Th1, whereas the response against peptide 129-40 was predominantly Th2-like[42]. Moreover, escaping variants of HCV epitope attenuate or fail to stimulate T-cell proliferation, which is accompanied by a shift in cytokine secretion patterns from one characteristic of a Th1 antiviral responses to a Th2 form[43]. Recent evidence suggests that the polymorphic nature of the MHC binding sites and differences in the T-cell repertoire among persons lead to highly variable binding affinity for the immunodominant HBV peptides, which in turn determines the outcome after acute HBV infection[44,45].
IL-12, a heterodimer composed of 2 subunits of p40 and p35 and secreted mainly by antigen-presenting cells (APC) such as activated macrophages and dendritic cells (DCs), is a crucial mediator between innate and adaptive immune responses. The transcriptional factor T-bet, which can induce transcription of an IFN-γ reporter gene and is specifically expressed in Th1 cells generated in the presence of IL-12[46], suppresses the expression of genes encoding IL-4, IL-5 and induces the synthesis of IFN-γ. These studies suggest that Th1 development process is governed by cytokine IL-12 to a great degree.
IL-12 is a key cytokine not only promoting Th1 - synergizing with IL-2, IL-12 induces rapid and efficient production of IFN-γ[47] by stimulation of the TCR-CD3 complex and activation of the CD28 receptor, but also maintaining Th1 responses[48] (Figure 1),-in that Th-cell differentiation is determined most probably early after infection by the balance between IL-12 and IL-10 ,IL-4, which favour Th1- and Th2-cell development, respectively. In addition, IL-12 correlates with virus clearance. In HBV-infected patients, a significant increase in IL-12 production was observed only in patients who cleared the virus. The peak of serum IL-12 associated with Th1 cytokines (IFN-γ) occurred after the ALT flare and preceded or coincided with the time of HBe seroconversion[8]. Thus, the findings in patients with chronic HBV infection support a proposed combination strategy for therapy with IL-12 plus vaccination[49,50]. After the ALT flare, the occurrence of IL-12 peak would be the reason that hepatocellular necrosis induced by CTL leads to the recruitment of macrophages and noncommitted T helper cells in the liver. Then the native particles of HBcAg are released from damaged hepatocytes and provide potent antigenic stimulation for these cells. In patients who are able to respond with an increase in IL-12 production, this will promote Th1 cell development and stimulate the production of IFN-γ and TNF-α, which will exert their noncytolytic antiviral effects.
IL-12 may be instrumental in the defense mechanism against HBV infection, and the elevation of its level can be indicative of hepatitis recovery[51]. The enhancing effect of IL-12 on IFN-γ production of PBMC in patients with chronic hepatitis B virus infection is increased during IFN-α treatment. Therefore, IFN-α and IL-12 may enhance the efficacy for the treatment of chronic HBV infection[52], and HCV-related cellular immune defect in patients with hepatitis C can be restored in most patients by IL-12[53]. However, Quiroga et al[54] found that HCV-infected patients with greater necro-inflammatory activity of liver showed greater IL-12 production by PBMC than those with minimal or mild activity and normal donors. Massive induction of the proinflammatory cytokines IL-12 and IFN-γ in liver specimens is apparently not counterbalanced by the anti-inflammatory cytokine IL-10, which may play an important role in promoting inflammatory reactions leading to massive liver damage in murine models of fulminant hepatitis B[55].
Macrophages can be segregated into two broad groups: resident tissue macrophages and inflammatory macrophages. Tissue macrophages are heterogeneous, and those isolated from different anatomical sites differ in function presumably because of adaptive responses to the local micro-environment[56]. Inflammatory macrophages are derived largely from circulating monocytes, which infiltrate damaged tissue, but some arise by local cell division[57]. There is now increasing evidence for the heterogeneity of macrophages that have infiltrated inflamed or otherwise damaged tissue, depending on the type and severity of injury, the stage of its evolution and the localization of the macrophages within the tissue[58].
One major function of macrophages is to provide a defense line against microbial invasion and to recognize and kill tumor cells. Macrophages can accomplish this in a direct manner, involving the release of products such as oxygen radicals and tumor necrosis factor that are harmful to microorganisms or cancer cells. On the other hand, they play an indirect role in these anti-microbial or anti-tumor activities by secretion of cytokines or by antigen processing and presentation, thereby regulating the immune system[59].
The macrophage presents HBV-derived proteins which activates CD4+T cells. The effects of stimulation on macrophages include increased cytokine production (TNF-α, IFN-γ, IL-1), expression of inducible nitric oxide synthase, nitric oxide secretion, and up-regulation of adhesion molecules. All these processes can lead, directly or indirectly, to increased cytotoxicity of the macrophages. We also found the higher expression level of granulate and activation - linked surface antigen CD69 by CD14 macrophage from peripheral blood in patients with chronic hepatitis than in controls[60].This activity was associated with high level transcription of IL-1, IL-6 and TNF-α (unpublished data). Probably, plasma HBV antigens activate macrophages from peripheral blood. Subsequently, such cytokines are produced. In addition, the increasing number of macrophage functions and heterogeneity in vitro and in vivo has led to the definition of macrophage activation states in parallel to the Th 1/2 paradigm. IFN-γ has long been known as the classical macrophage activating factor inducing cytokine secretion by macrophages supporting Th1-driven immune responses. IL-4 which was historically regarded as macrophage deactivators is now thought to induce alternative immunological activation of macrophages[9], in that it enhances of the capacity of macrophages for endocytosis and antigen presentation by the induction of mannose receptor expression[10]. In normal immunological process, classical and alternative macrophage activation maintains the balance of macrophage.
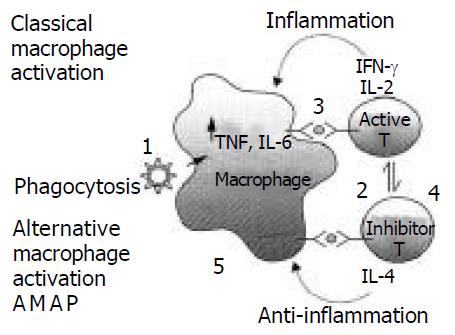
Figure 2 shows a macrophage activation cycle wherein multiple steps occur during various forms of activation and recycling of macrophage function so as to achieve balanced macrophage activation (Steps 1-5)[61]. When hepatitis virus invades the body, tissue-resident macrophages undergo local activation and engulf the virus or antigen and enhanced recruitment of monocytes and precursors from bone-marrow pools results in the accumulation of tissue macrophages that have enhanced turnover and an altered phenotype[62].After the antigen presentation, Th cells are activated through MHC class II. Th1 (stand for active Th) cell produces IL-2 and IFN-γ and Th2 (stand for inhibitor T) cell secrets IL-4 and IL-10, which is involved in B cell activation as well as providing signals for balanced macrophage activation. Production of IL-4 is known to activate the alternative macrophage activation pathway[10] (step 5). Although IL-4 induces mannose receptor expression and enhance the capacity of macrophages for endocytosis and antigen presentation[62], alternative pathway activated macrophages in vitro actively inhibit mitogen-induced proliferation of peripheral blood lymphocytes[63] and CD4+T cells[64-66] These findings convincingly confirm that alternative activation generates immunosuppressive macrophage populations. In fact, co-induction of IL-10 with IL-4 secreted by Th2 cells, mainly contributes to the inhibition effect. The net result of excess IL-10 production shuts off the Th1 activation pathway[61]. Therefore, the balance of macrophage and these cytokines are closely related to viral infectious diseases such as AIDS and viral hepatitis.
Macrophages may contribute either directly or indirectly to the hepatonecrosis with fulminant virus infection[67] through classical macrophage pathway. Macrophages in the liver called Kupffer cells activate Th1 (IFN-γ), IFN-γ will activate Kupffer cells[68,69]. This results in Kupffer overactivation, which in turn promotes cytokines production (TNF-α, IFN-γ), expression of inducible NOS (iNOS), nitric oxide (NO) secretion. All these processes can lead, directly or indirectly, to increased cytotoxicity of the macrophages-this is the classical macrophage activation pathway.
IL-12 is a cytokine secreted by APCs such as activated macrophages and DCs[70]. It has an important role against intracellular pathogens by promoting Th1 cell development, cell mediated cytotoxicity and IFN-γ production[71,72]. For example, a significant increase in IL-12 production was observed only in patients with chronic hepatitis C who cleared the virus[8]. On the one hand, IFN-γ, activating macrophage and TNF-α secreted by macrophage, exert their noncytolytic antiviral effects. On the other hand, macrophage kills small fraction of infected hepatocytes. As mentioned above, the increase of serum IL-12 and Th1 cytokines always followed the ALT flare[8], confirming the function of IL-12 in promoting Th1 cell development, and binary function of macrophage. This also explains that HCV-infected patients with greater necro-inflammatory activity of liver showed greater IL-12 production by PBMC than those with minimal or mild activity in normal donors, and why Th1 predominance in HCV infection was correlated with the direct cytotoxic effect[41,21] and the inhibition of viral replication.
The greater production of IL-12 associated with greater necro-inflammatory activity of liver in HCV-infected patients[54] and response of IFN-γ to HCV core protein with chronic liver disease suggest a cellular immune response to the onset of the necroinflammatory process of hepatitis[35]. In murine model of fulminant hepatitis B, massive liver damage was associated with the massive induction of IL-12 and IFN-γ in liver specimens. Probably, the proinflammatory cytokines are apparently not counterbalanced by the anti-inflammatory cytokine IL-10. Thus IL-12 may play an important role in promoting inflammatory reactions[55]. These cases suggest that the macrophages are in the hyperactive situation, probably due to the imbalance of Th1/Th2 and failing to establish the alternative macrophage activation, which results in the imbalance of macrophage. Thus massive hepatocytes are killed by macrophages or overactivited CTLs.
The steps 2 and 3 in Figure 2 are continually stimulated when foreign virus can not be cleared by successful immune response for Lack of optimal T-cell reactivity that would reestablish balanced macrophage activation. The immunologic overstimulation would predictably lead to pathologic sequelae such as cirrhosis and hepatoma in chronic hepatitis B and C infections[73]
After long periods of time during which steps 2 and 3 are overemphasized, there would be a predicted shortage of cells to accomplish steps 5 and 1.There would also be an initial overdrive of Th1 cell population. Patients with HIV also have been observed to have a dramatic Th1 to Th2 shift as described in step 4 and patients with chronic hepatitis B appeared to be Th2 predominant[30]. The balanced macrophage activation theory predicts that this shift is compensatory in nature with the T cells attempting to regulate balanced macrophage activation through production of IL-4 which induces step5. Th2 predominance would be suffered by patients with chronic viral disease, which would cause secondary immunopathogenic changes, such as HCV-related liver cirrhosis(HCC)[74], while patients with histology of inflammation showed a significantly higher CD4+Th1 response to the HCV core antigen as compared to patients with histology of fibrosis/cirrhosis[75]. These findings suggest that Th1/Th2 imbalance in HCV-related cirrhosis would decrease the antitumor immunity and its improvement might present the protective effect from HCC[74]. At the same time, there exists the exhaustion of cells in steps 5 and 1.This would decrease the rate of phagecytosis.
Alternative macrophage pathway (step 4) has the following features: (1) the production of angiogenic factors, (2) inhibition of T cell responses, (3) associated downregulation of inflammatory-mediator production characteristic of classical activated macrophages, and (4) with the alternative macrophage activation chemokine-1 (AMAC-1)[76], also known as macrophage inflammatory protein-4 (MIP-4). But few studies were performed in aspect of macrophage, and little is known about the mechanism of the balanced macrophage theory in chronic virus liver infection.
Recent data have made a major shift in the role of macrophages in HIV[77] and inflammatory kidney disease. They can no longer be regarded solely as causing injury but rather as cells that can also promote resolution[78,79]. This means that strategies to prevent macrophage influx may be beneficial to patients with hepatitis. Future experiments will need to define methods for determining the functional attributes of macrophages in clinical liver biopsies, and effective ways to manipulate the function of inflammatory macrophages in vivo.
Given the diverse range of functions macrophages can assume, it becomes possible to modulate disease by altering macrophage activity. The classic view would be that the overall inflammatory environment is a balance between pro- and anti-inflammatory cytokines, determining infiltrating and resident cell function. An increase of pro-inflammatory cytokines from macrophages such as TNF-α or IL-1β thus worsens inflammation, whereas antagonists of these molecules such as IL-1 receptor antagonist (IL-1ra), IL-1 type II decoy receptor (IL-1RII) and soluble TNF receptor result in reduced injury[80]. A number of cytokines are described as anti-inflammatory, including the Th2 cytokines IL-4, IL-10 and IL-13, IL-6 and TGF-β. rIL-10 treatment of patients with advanced fibrosis who had failed antiviral therapy appeared to decrease disease activity[81]. TGF-β2 significantly suppressed IFN-γ production at the single-cell level, indicating that the enhanced down-regulation of Th1 by TGF-β2 in patients with HCVrelated liver cirrhosis might be effective against hepatoma[82]. But not all anti-inflammatory cytokines are equal in their ability to modulate macrophage function in vivo. In contrast to the results with the Th2 cytokines, IL-4 and IL-10, infusions of TGF-β do not modulate inflammatory macrophage function in glomerulonephritis. For example, Infusion of TGF-β3 in rats with NTN (nephrotoxic nephritis RANTES) before the onset of disease did not alter the degree of proteinuria, although the number of infiltrating macrophages was reduced[83]. The study about TGF-β treatment of animal models and patients with hepatitis is still unknown.
Advanced clinical studies of WF10 (completely blocked antigen activation of T cells responsiveness -in step 2) are currently underway in the USA for treatment of patients with HIV disease. The patients who received two cycles of WF10 showed chronic immunological changes by downregulating inflammatory macrophages and reestablished alternative macrophage activation, which was consistent with induction of balanced macrophage activation[84]. Similarly, it is possible to modify inflammatory virus liver disease using a range of cytokines (Th1/Th2 types) with well-defined effects on macrophage activation. For example, defects in T cell response have been suspected as a major factor involved in the pathogenesis of chronic hepatitis B[11-15], and favoring Th2 type cytokine may lead to chronic infections with HBV. Recent study has shown that activation of Th1 immunity accompanied by enhancement of CTL activity during therapy is a common immune mechanism for the successful treatment of hepatitis B and C[85]. Using HBV core gene transduced DCs as APCs, HBcAg specific CTLs and Th1 type immune responses could be generated in the mice, which would be a new way to deal with the viral hepatitis[86].Thus, treatment with IL-12 to drive T cell reactivity[87], and usage of IL-4 receptor antagonist are probably the practicable way to clear virus and remove HBV-infected hepatocytes. Although IL-12 as monotherapy in patients with HCV did not alter the production of regulatory cytokines produced by Th1/2 cells[88] and had low efficacy[89,90], IL-12 combining with IFN-α may enhance the efficacy for the treatment of chronic hepatitis B virus infection[52]and may be a predisposition for elimination of HBeAg and successful treatment of hepatitis B[91].
Edited by Liu HX and Xu FM
| 1. | Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348-2357. [PubMed] |
| 2. | Romagnani S, Parronchi P, D'Elios MM, Romagnani P, Annunziato F, Piccinni MP, Manetti R, Sampognaro S, Mavilia C, De Carli M. An update on human Th1 and Th2 cells. Int Arch Allergy Immunol. 1997;113:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 83] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Racke MK, Bonomo A, Scott DE, Cannella B, Levine A, Raine CS, Shevach EM, Röcken M. Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J Exp Med. 1994;180:1961-1966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 478] [Cited by in RCA: 473] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 4. | Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3292] [Cited by in RCA: 3186] [Article Influence: 109.9] [Reference Citation Analysis (0)] |
| 5. | Clerici M, Shearer GM. The Th1-Th2 hypothesis of HIV infection: new insights. Immunol Today. 1994;15:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 487] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 6. | Yamamura M, Uyemura K, Deans RJ, Weinberg K, Rea TH, Bloom BR, Modlin RL. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 695] [Cited by in RCA: 718] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 7. | Sher A, Coffman RL. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu Rev Immunol. 1992;10:385-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 590] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 8. | Rossol S, Marinos G, Carucci P, Singer MV, Williams R, Naoumov NV. Interleukin-12 induction of Th1 cytokines is important for viral clearance in chronic hepatitis B. J Clin Invest. 1997;99:3025-3033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 176] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Birk RW, Gratchev A, Hakiy N, Politz O, Schledzewski K, Guillot P, Orfanos CE, Goerdt S. [Alternative activation of antigen-presenting cells: concepts and clinical relevance]. Hautarzt. 2001;52:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Montaner LJ, da Silva RP, Sun J, Sutterwala S, Hollinshead M, Vaux D, Gordon S. Type 1 and type 2 cytokine regulation of macrophage endocytosis: differential activation by IL-4/IL-13 as opposed to IFN-γ or IL-10. J Immunol. 1999;162:4606-4613. [PubMed] |
| 11. | Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1201] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 12. | Ishikawa T, Kakumu S, Yoshioka K, Wakita T, Takayanagi M, Olido E. Immune response of peripheral blood mononuclear cells to antigenic determinants within hepatitis B core antigen in HB virus-infected man. Liver. 1992;12:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Marinos G, Torre F, Chokshi S, Hussain M, Clarke BE, Rowlands DJ, Eddleston AL, Naoumov NV, Williams R. Induction of T-helper cell response to hepatitis B core antigen in chronic hepatitis B: a major factor in activation of the host immune response to the hepatitis B virus. Hepatology. 1995;22:1040-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Ferrari C, Penna A, Bertoletti A, Valli A, Antoni AD, Giuberti T, Cavalli A, Petit MA, Fiaccadori F. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J Immunol. 1990;145:3442-3449. [PubMed] |
| 15. | Löhr HF, Weber W, Schlaak J, Goergen B, Meyer zum Buschenfelde KH, Gerken G. Proliferative response of CD4+ T cells and hepatitis B virus clearance in chronic hepatitis with or without hepatitis B e-minus hepatitis B virus mutants. Hepatology. 1995;22:61-68. [PubMed] |
| 16. | Toyonaga T, Hino O, Sugai S, Wakasugi S, Abe K, Shichiri M, Yamamura K. Chronic active hepatitis in transgenic mice expressing interferon-gamma in the liver. Proc Natl Acad Sci USA. 1994;91:614-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 156] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Peters M. Actions of cytokines on the immune response and viral interactions: an overview. Hepatology. 1996;23:909-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Ando K, Moriyama T, Guidotti LG, Wirth S, Schreiber RD, Schlicht HJ, Huang SN, Chisari FV. Mechanisms of class I restricted immunopathology. A transgenic mouse model of fulminant hepatitis. J Exp Med. 1993;178:1541-1554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 327] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 19. | Guidotti LG, Ando K, Hobbs MV, Ishikawa T, Runkel L, Schreiber RD, Chisari FV. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc Natl Acad Sci USA. 1994;91:3764-3768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 319] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 20. | Nayersina R, Fowler P, Guilhot S, Missale G, Cerny A, Schlicht HJ, Vitiello A, Chesnut R, Person JL, Redeker AG. HLA A2 restricted cytotoxic T lymphocyte responses to multiple hepatitis B surface antigen epitopes during hepatitis B virus infection. J Immunol. 1993;150:4659-4671. [PubMed] |
| 21. | Barnaba V, Franco A, Paroli M, Benvenuto R, De Petrillo G, Burgio VL, Santilio I, Balsano C, Bonavita MS, Cappelli G. Selective expansion of cytotoxic T lymphocytes with a CD4+CD56+ surface phenotype and a T helper type 1 profile of cytokine secretion in the liver of patients chronically infected with Hepatitis B virus. J Immunol. 1994;152:3074-3087. [PubMed] |
| 22. | Clerici M, Hakim FT, Venzon DJ, Blatt S, Hendrix CW, Wynn TA, Shearer GM. Changes in interleukin-2 and interleukin-4 production in asymptomatic, human immunodeficiency virus-seropositive individuals. J Clin Invest. 1993;91:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 346] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 23. | Chisari FV. Hepatitis B virus transgenic mice: insights into the virus and the disease. Hepatology. 1995;22:1316-1325. [PubMed] |
| 24. | Ando K, Guidotti LG, Wirth S, Ishikawa T, Missale G, Moriyama T, Schreiber RD, Schlicht HJ, Huang SN, Chisari FV. Class I-restricted cytotoxic T lymphocytes are directly cytopathic for their target cells in vivo. J Immunol. 1994;152:3245-3253. [PubMed] |
| 25. | Gilles PN, Fey G, Chisari FV. Tumor necrosis factor alpha negatively regulates hepatitis B virus gene expression in transgenic mice. J Virol. 1992;66:3955-3960. [PubMed] |
| 26. | Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 840] [Article Influence: 29.0] [Reference Citation Analysis (1)] |
| 27. | Jilbert AR, Wu TT, England JM, Hall PM, Carp NZ, O'Connell AP, Mason WS. Rapid resolution of duck hepatitis B virus infections occurs after massive hepatocellular involvement. J Virol. 1992;66:1377-1388. [PubMed] |
| 28. | Jiang R, Lu Q, Hou J. [Polarized populations of T helper cells in patients with chronic hepatitis B virus infection]. Zhonghua Yi Xue Za Zhi. 2000;80:741-744. [PubMed] |
| 29. | Maggi E, Mazzetti M, Ravina A, Annunziato F, de Carli M, Piccinni MP, Manetti R, Carbonari M, Pesce AM, del Prete G. Ability of HIV to promote a TH1 to TH0 shift and to replicate preferentially in TH2 and TH0 cells. Science. 1994;265:244-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 350] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 30. | Lee M, Lee M, Lee SK, Son M, Cho SW, Park S, Kim HI. Expression of Th1 and Th2 type cytokines responding to HBsAg and HBxAg in chronic hepatitis B patients. J Korean Med Sci. 1999;14:175-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Jiang R, Feng X, Guo Y, Lu Q, Hou J, Luo K, Fu N. T helper cells in patients with chronic hepatitis B virus infection. Chin Med J (Engl). 2002;115:422-424. [PubMed] |
| 32. | Jacobson Brown PM, Neuman MG. Immunopathogenesis of hepatitis C viral infection: Th1/Th2 responses and the role of cytokines. Clin Biochem. 2001;34:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Fan XG, Liu WE, Li CZ, Wang ZC, Luo LX, Tan DM, Hu GL, Zhang Z. Circulating Th1 and Th2 cytokines in patients with hepatitis C virus infection. Mediators Inflamm. 1998;7:295-297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Kobayashi K, Ishii M, Igarashi T, Satoh T, Miyazaki Y, Yajima Y, Ukai K, Suzuki H, Kanno A, Ueno Y. Profiles of cytokines produced by CD4-positive T lymphocytes stimulated by anti-CD3 antibody in patients with chronic hepatitis C. J Gastroenterol. 1998;33:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Iwata K, Wakita T, Okumura A, Yoshioka K, Takayanagi M, Wands JR, Kakumu S. Interferon gamma production by peripheral blood lymphocytes to hepatitis C virus core protein in chronic hepatitis C infection. Hepatology. 1995;22:1057-1064. [PubMed] |
| 36. | Bergamini A, Bolacchi F, Cerasari G, Carvelli C, Faggioli E, Cepparulo M, Demin F, Uccella I, Bongiovanni B, Niutta P. Lack of evidence for the Th2 predominance in patients with chronic hepatitis C. Clin Exp Immunol. 2001;123:451-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Sobue S, Nomura T, Ishikawa T, Ito S, Saso K, Ohara H, Joh T, Itoh M, Kakumu S. Th1/Th2 cytokine profiles and their relationship to clinical features in patients with chronic hepatitis C virus infection. J Gastroenterol. 2001;36:544-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Tran A, Yang G, Doglio A, Ticchioni M, Laffont C, Durant J, Bernard JL, Gugenheim J, Saint-Paul MC, Bernard A. Phenotyping of intrahepatic and peripheral blood lymphocytes in patients with chronic hepatitis C. Dig Dis Sci. 1997;42:2495-2500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Khakoo SI, Soni PN, Savage K, Brown D, Dhillon AP, Poulter LW, Dusheiko GM. Lymphocyte and macrophage phenotypes in chronic hepatitis C infection. Correlation with disease activity. Am J Pathol. 1997;150:963-970. [PubMed] |
| 40. | Picker LJ, Singh MK, Zdraveski Z, Treer JR, Waldrop SL, Bergstresser PR, Maino VC. Direct demonstration of cytokine synthesis heterogeneity among human memory/effector T cells by flow cytometry. Blood. 1995;86:1408-1419. [PubMed] |
| 41. | Minutello MA, Pileri P, Unutmaz D, Censini S, Kuo G, Houghton M, Brunetto MR, Bonino F, Abrignani S. Compartmentalization of T lymphocytes to the site of disease: intrahepatic CD4+ T cells specific for the protein NS4 of hepatitis C virus in patients with chronic hepatitis C. J Exp Med. 1993;178:17-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 174] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 42. | Milich DR, Schödel F, Peterson DL, Jones JE, Hughes JL. Characterization of self-reactive T cells that evade tolerance in hepatitis Be antigen transgenic mice. Eur J Immunol. 1995;25:1663-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Wang JH, Layden TJ, Eckels DD. Modulation of the peripheral T-Cell response by CD4 mutants of hepatitis C virus: transition from a Th1 to a Th2 response. Hum Immunol. 2003;64:662-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Ferrari C, Bertoletti A, Penna A, Cavalli A, Valli A, Missale G, Pilli M, Fowler P, Giuberti T, Chisari FV. Identification of immunodominant T cell epitopes of the hepatitis B virus nucleocapsid antigen. J Clin Invest. 1991;88:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 171] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 45. | Thursz MR, Kwiatkowski D, Allsopp CE, Greenwood BM, Thomas HC, Hill AV. Association between an MHC class II allele and clearance of hepatitis B virus in the Gambia. N Engl J Med. 1995;332:1065-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 317] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 46. | Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2577] [Cited by in RCA: 2716] [Article Influence: 108.6] [Reference Citation Analysis (0)] |
| 47. | Chan SH, Kobayashi M, Santoli D, Perussia B, Trinchieri G. Mechanisms of IFN-γ induction by natural killer cell stimulatory factor (NKSF/IL-12). Role of transcription and mRNA stability in the synergistic interaction between NKSF and IL-2. J Immunol. 1992;148:92-98. [PubMed] |
| 48. | Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2687] [Cited by in RCA: 2875] [Article Influence: 130.7] [Reference Citation Analysis (0)] |
| 49. | Milich DR, Wolf SF, Hughes JL, Jones JE. Interleukin 12 suppresses autoantibody production by reversing helper T-cell phenotype in hepatitis B e antigen transgenic mice. Proc Natl Acad Sci USA. 1995;92:6847-6851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Gherardi MM, Ramírez JC, Esteban M. Towards a new generation of vaccines: the cytokine IL-12 as an adjuvant to enhance cellular immune responses to pathogens during prime-booster vaccination regimens. Histol Histopathol. 2001;16:655-667. [PubMed] |
| 51. | Liu Q, Feng GX, Lin YL, Peng YZ, Mo BQ. Detection of interleukin-6 and -12 in of hepatitis B patients and its clinical significance. Di Yi Jun Yi Da Xue Xue Bao. 2001;21:858-859. [PubMed] |
| 52. | Wang S, Lin Y, Ma W, Zhang B, Qi S, Lan F. [Effect of IL-12 on IFN-gamma and IL-10 produced by peripheral blood mononuclear cells in patients with chronic hepatitis B virus infection during IFN-alpha treatment]. Zhonghua Gan Zang Bing Za Zhi. 2002;10:116-119. [PubMed] |
| 53. | Schlaak JF, Pitz T, Löhr HF, Meyer zum Büschenfelde KH, Gerken G. Interleukin 12 enhances deficient HCV-antigen-induced Th1-type immune response of peripheral blood mononuclear cells. J Med Virol. 1998;56:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 54. | Quiroga JA, Martín J, Navas S, Carreño V. Induction of interleukin-12 production in chronic hepatitis C virus infection correlates with the hepatocellular damage. J Infect Dis. 1998;178:247-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Leifeld L, Cheng S, Ramakers J, Dumoulin FL, Trautwein C, Sauerbruch T, Spengler U. Imbalanced intrahepatic expression of interleukin 12, interferon gamma, and interleukin 10 in fulminant hepatitis B. Hepatology. 2002;36:1001-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 56. | Henson PM, Riches DW. Modulation of macrophage maturation by cytokines and lipid mediators: a potential role in resolution of pulmonary inflammation. Ann N Y Acad Sci. 1994;725:298-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 57. | Yang N, Isbel NM, Nikolic-Paterson DJ, Li Y, Ye R, Atkins RC, Lan HY. Local macrophage proliferation in human glomerulonephritis. Kidney Int. 1998;54:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 128] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 58. | Segerer S, MacK M, Regele H, Kerjaschki D, Schlöndorff D. Expression of the C-C chemokine receptor 5 in human kidney diseases. Kidney Int. 1999;56:52-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 123] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 59. | Klimp AH, de Vries EG, Scherphof GL, Daemen T. A potential role of macrophage activation in the treatment of cancer. Crit Rev Oncol Hematol. 2002;44:143-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 239] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 60. | Wel R, Chen B, Gan TF, Zhou XM, Zhang YQ, Ren DL. Appli-cation of flow cytometry to analyzing the activation states of peripheral blood mononuclear cells CD14 macrophage. Chin J Lab Med. 2003;26:1-4. |
| 61. | McGrath MS, Kodelja V. Balanced macrophage activation hypothesis: a biological model for development of drugs targeted at macrophage functional states. Pathobiology. 1999;67:277-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 62. | Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4403] [Cited by in RCA: 4595] [Article Influence: 208.9] [Reference Citation Analysis (0)] |
| 63. | Wang J, Crawford K, Yuan M, Wang H, Gorry PR, Gabuzda D. Regulation of CC chemokine receptor 5 and CD4 expression and human immunodeficiency virus type 1 replication in human macrophages and microglia by T helper type 2 cytokines. J Infect Dis. 2002;185:885-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Kodelja V, Müller C, Tenorio S, Schebesch C, Orfanos CE, Goerdt S. Differences in angiogenic potential of classically vs alternatively activated macrophages. Immunobiology. 1997;197:478-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 141] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 65. | Saha B, Das G, Vohra H, Ganguly NK, Mishra GC. Macrophage-T cell interaction in experimental mycobacterial infection. Selective regulation of co-stimulatory molecules on Mycobacterium-infected macrophages and its implication in the suppression of cell-mediated immune response. Eur J Immunol. 1994;24:2618-2624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 66. | Kirschmann DA, He X, Murasko DM. Inhibition of macrophage-induced, antigen-specific T-cell proliferation by poly I: C role of suppressor macrophages. Immunology. 1994;82:238-243. [PubMed] |
| 67. | Liu M, Chan CW, McGilvray I, Ning Q, Levy GA. Fulminant viral hepatitis: molecular and cellular basis, and clinical implications. Expert Rev Mol Med. 2001;2001:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 68. | Shiratori Y, Takikawa H, Kawase T, Sugimoto T. Superoxide anion generating capacity and lysosomal enzyme activities of Kupffer cells in galactosamine induced hepatitis. Gastroenterol Jpn. 1986;21:135-144. [PubMed] |
| 69. | Andus T, Bauer J, Gerok W. Effects of cytokines on the liver. Hepatology. 1991;13:364-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 234] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 70. | D'Andrea A, Rengaraju M, Valiante NM, Chehimi J, Kubin M, Aste M, Chan SH, Kobayashi M, Young D, Nickbarg E. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387-1398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 809] [Cited by in RCA: 906] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 71. | Park AY, Hondowicz BD, Scott P. IL-12 is required to maintain a Th1 response during Leishmania major infection. J Immunol. 2000;165:896-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 163] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 72. | Yap G, Pesin M, Sher A. Cutting edge: IL-12 is required for the maintenance of IFN-gamma production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J Immunol. 2000;165:628-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 224] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 73. | Chen YP, Feng XR, Dai L, Ding HB, Zhang L. [Screening and evaluation of non-invasive diagnosis markers for compensated liver cirrhosis in patients with chronic hepatitis B]. Zhonghua Ganzangbing Zazhi. 2003;11:225-227. [PubMed] |
| 74. | Sakaguchi E, Kayano K, Segawa M, Aoyagi M, Sakaida I, Okita K. [Th1/Th2 imbalance in HCV-related liver cirrhosis]. Nihon Rinsho. 2001;59:1259-1263. [PubMed] |
| 75. | Sreenarasimhaiah J, Jaramillo A, Crippin J, Lisker-Melman M, Chapman WC, Mohanakumar T. Lack of optimal T-cell reactivity against the hepatitis C virus is associated with the development of fibrosis/cirrhosis during chronic hepatitis. Hum Immunol. 2003;64:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 76. | Kitching AR, Tipping PG, Mutch DA, Huang XR, Holdsworth SR. Interleukin-4 deficiency enhances Th1 responses and crescentic glomerulonephritis in mice. Kidney Int. 1998;53:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 77. | Raffanti SP, Schaffner W, Federspiel CF, Blackwell RB, Ching OA, Kühne FW. Randomized, double-blind, placebo-controlled trial of the immune modulator WF10 in patients with advanced AIDS. Infection. 1998;26:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 78. | Rovin BH. Chemokine blockade as a therapy for renal disease. Curr Opin Nephrol Hypertens. 2000;9:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 79. | Erwig LP, Stewart K, Rees AJ. Macrophages from inflamed but not normal glomeruli are unresponsive to anti-inflammatory cytokines. Am J Pathol. 2000;156:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Kluth DC, Rees AJ. New approaches to modify glomerular inflammation. J Nephrol. 1999;12:66-75. [PubMed] |
| 81. | Nelson DR, Tu Z, Soldevila-Pico C, Abdelmalek M, Zhu H, Xu YL, Cabrera R, Liu C, Davis GL. Long-term interleukin 10 therapy in chronic hepatitis C patients has a proviral and anti-inflammatory effect. Hepatology. 2003;38:859-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 82. | Sakaguchi E, Kayano K, Segawa M, Okamoto M, Sakaida I, Okita K. Th1 down-regulation at the single-lymphocyte level in HCV-related liver cirrhosis and the effect of TGF-beta on Th1 response: possible implications for the development of hepatoma. Hepatol Res. 2002;24:282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 83. | Wilson HM, Minto AW, Brown PA, Erwig LP, Rees AJ. Transforming growth factor-beta isoforms and glomerular injury in nephrotoxic nephritis. Kidney Int. 2000;57:2434-2444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 84. | McGrath MS, Benike C, Kuehne FW, Engleman E. Effect of WF10 (TCDO) on antigen presentation. Transplant Proc. 1998;30:4200-4204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 85. | Tsai SL, Sheen IS, Chien RN, Chu CM, Huang HC, Chuang YL, Lee TH, Liao SK, Lin CL, Kuo GC. Activation of Th1 immunity is a common immune mechanism for the successful treatment of hepatitis B and C: tetramer assay and therapeutic implications. J Biomed Sci. 2003;10:120-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 86. | Ding CL, Yao K, Zhang TT, Zhou F, Xu L, Xu JY. Generation of cytotoxic T cell against HBcAg using retrovirally transduced dendritic cells. World J Gastroenterol. 2003;9:1512-1515. [PubMed] |
| 87. | Teuber G, Rossol S, Lee JH, Dietrich CF, Zeuzem S. TH1/TH2 serum cytokine profiles and soluble TNF-receptor response in patients with chronic hepatitis C during recombinant human interleukin-12 (rHuIL-12) treatment. Z Gastroenterol. 2002;40:487-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 88. | Barth H, Klein R, Berg PA, Wiedenmann B, Hopf U, Berg T. Analysis of the effect of IL-12 therapy on immunoregulatory T-cell subsets in patients with chronic hepatitis C infection. Hepatogastroenterology. 2003;50:201-206. [PubMed] |
| 89. | Pockros PJ, Patel K, O'Brien C, Tong M, Smith C, Rustgi V, Carithers RL, McHutchison JG, Olek E, Debruin MF. A multicenter study of recombinant human interleukin 12 for the treatment of chronic hepatitis C virus infection in patients nonresponsive to previous therapy. Hepatology. 2003;37:1368-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 90. | Zeuzem S, Carreño V. Interleukin-12 in the treatment of chronic hepatitis B and C. Antiviral Res. 2001;52:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 91. | Barth H, Klein R, Berg PA, Wiedenmann B, Hopf U, Berg T. Induction of T helper cell type 1 response and elimination of HBeAg during treatment with IL-12 in a patient with therapy-refractory chronic hepatitis B. Hepatogastroenterology. 2001;48:553-555. [PubMed] |