INTRODUCTION
Homocysteine (Hcy) is a toxic non-protein sulfur containing amino acids in humans. It is formed exclusively upon demethylation of the essential amino acid-methionine. Hcy is metabolized either through remethylation or transsulfuration pathways and is nutritionally regulated. Normal concentrations of total homocysteine in plasma are in the range of 5 to 16 μmol/L and the desired upper limit for Hcy concentration should be 10 μmol/L. An elevated plasma Hcy level is denoted hyperhomocysteinemia (HHcy). Three ranges of HHcy are defined: moderate (16 to 30 μmol/L), intermediate (31 to 100 μmol/L), and severe (> 100 μmol/L). Individuals who consume a large amount of food rich in animal protein may ingest two to three grams of methionine, resulting in postprandial Hcy concentrations greater than 20 μmol/L. Clinical HHcy was first described more than 40 years ago in children with learning difficulties[1-3], and it has since been estimated that moderate HHcy occurs in 5%-7% of the general population. Evidence now indicates that moderate HHcy is an important and independent risk factor for several disorders, including atherosclerosis, diabetes, fatty liver, immune activation, and neurodegenerations such as Alzheimer’s and Parkinson’s diseases[3-9].
Readers are referred to recent reviews on HHcy and functions of Hcy[10,11]. The main goal of this article is to provide information on major causes of HHcy, potential mechanisms of Hcy toxicity, with emphasis on endoplasmic reticulum (ER) stress mechanism, and animal models for the study of biological effects of HHcy. We would also summarize our ongoing work on ethanol-induced HHcy and liver injury in an intragastric ethanol fed murine model.
HCY METABOLISM AND HHCY
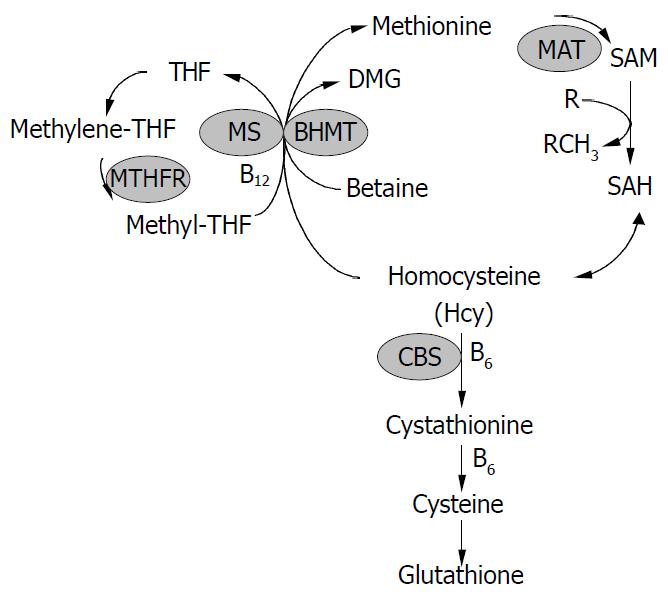
Hcy was formed from methionine after removal of the methyl group on S-adenosylmethionine (SAM) (Figure 1). Hcy metabolism involves reversible formation of S-adenosylhomocysteine (SAH), remethylation to methionine by betaine-homocysteine methyltransferase (BHMT) (liver and kidney restricted), which is vitamin-independent, and by the ubiquitous methionine synthase (MS), which is dependent on vitamin B12 and methylenetetrahydrofolate (MTHF) production via 5, 10-methylenetetrahydrofolate reductase (MTHFR). Hcy can also be converted through transsulfuration to cystathionine for the formation of cysteine and glutathione (GSH). The transsulfuration is catalyzed by cystathionine-β-synthase (CBS) and is dependent on vitamin B6. In addition, Hcy can be converted to Hcy-tRNA. Although it was not incorporated into protein due to editing mechanisms, nitroso-Hcy-tRNA is stable and might play a role in Hcy-induced protein misfolding along with the formation of Hcy-protein-SH mixed disulfides and Hcy thiolactone covalent binding to lysine amino groups[11]. Hcy-t-RNA is edited through the action of methionyl-t-RNA synthetase (ATP consuming) by formation of a thioester thiolactone which could covalently bind to protein amino groups. Thus, homocysteinylation of proteins depends on the formation of thiolactone[12,13].
Figure 1 Homocysteine metabolism.
Homocysteine has three main metabolic fates: to be remethylated to methionine, to en-ter the cysteine biosynthetic pathway, and to be released into the extracellular medium. CBS, cystathionine -synthase; MS, methionine synthase; THF, tetrahydrofolate; MTHFR, 5, 10-methylenetetrahydrofolate reductase; BHMT, betaine-ho-mocysteine methyltransferase; DMG, dimethylglycine; MAT, methionine adenosyltransferase; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine.
Tight regulation of Hcy metabolism depends on different affinities of MS, BHMT, and CBS for Hcy. MS and BHMT show low Km values for Hcy (< 0.1 mmol/L), and CBS has high Km values for Hcy (> 1 mmol/L). At low Hcy concentrations, methionine conservation was favored; and at high Hcy concentrations, immediate and long-term drainage of Hcy via the transsulfuration pathway was ensured[14]. SAM could play a key regulatory role by allosterically inhibiting MTHFR and BHMT and activating CBS[15-18]. Thus, SAM may be a regulatory switch in Hcy metabolism: low SAM favors remethylation and conservation of Hcy for methionine synthesis, whereas high levels favor transsulfuration. High Hcy levels can decrease the SAM/SAH ratio, since most methyltransferases bind to SAH with higher affinity than SAM, elevated SAH inhibits methylation. In vitro, under “physiological” conditions of concentrated 27 000 g postmitochondrial supernatant with 8 mmol/L GSH, 0.3 mmol/L serine, 2 mmol/L betaine, 60 μmol/L methionine, 50 μmol/L methyl THF, 60 μmol/L SAM and 10 μmol/L SAH, transsulfuration accounted for 46% of Hcy metabolism and the remainder was equally contributed to by MS and BHMT. The need to conserve methionine (e.g. low protein diet) resulted in decreased cystathionine production and increased Hcy remethylation. Conversely, in the presence of excess methionine, SAM activated the cystathionine pathway.
HHcy results from increased levels of intracellular Hcy that is readily released into the extracellular medium: plasma or body fluid. Kidney might be a major site for the removal and metabolism of Hcy primarily through the transsulfuration pathway[19]. Renal impairment often causes HHcy, reflecting a role of kidney in Hcy clearance from plasma. This fact might contribute to the high incidence of vascular complications in patients with chronic renal failure[20]. Genetic abnormalities, age, sex and various nutritional and hormonal determinants contribute to HHcy. However, genetic and nutritional disorders are the major factors. Genetic disorders involve polymorphism in the genes coding for MTHFR and CBS. The most common genetic defect associated with mild HHcy is a point mutation, namely, a C to T substitution at nucleotide 677 (C677→T) in the open reading frame of the gene for MTHFR. This point mutation could cause a substitution of valine for alanine in the functional enzyme[21], resulting in a thermolabile variant of the enzyme with decreased total activity. This is an autosomal recessive mutation, and the frequency of the C677→T polymorphism varied among racial and ethnic groups, with 13% of T/T homozygous and 50% C/T heterozygous among Caucasian and Asian populations, and very low incidence among African-Americans[21-27]. Premature atherosclerosis and thrombotic disease were observed in MTHFR deficiency[23-28]. The most common genetic cause associated with severe HHcy is homozygous CBS deficiency, which resulted in plasma Hcy concentrations of up to 400 μmol/L, compared to normal plasma levels of 10 μmol/L[28-30]. Homozygous CBS deficiency, T833→C and G919→A mutations, were inherited as an autosomal recessive disorder with pleiotropic clinical manifestations, including mental retardation, ectopia lentis, osteoporosis, skeletal abnormalities and hepatic steatosis[28-30]. Patients were usually at higher risk for premature atherosclerosis and thrombotic disease, which is the major cause of death[31-33]. CBS deficiency has a worldwide incidence of 1:344 000 live births, ranging from 1:58 000 to 1:1 000 000 in countries that perform newborn screening[31]. While homozygous CBS deficiency is rare, heterozygous CBS deficiency occurs in approximately 1% of the general population and is associated with premature atherosclerosis and thrombotic disease in phenotypically normal individuals[31-33].
Nutritional disorders that potentially lead to HHcy include deficiencies in vitamin B12, folate and vitamin B6, as the de novo synthesis of methionine methyl groups requires both vitamin B12 and folate cofactors whereas the synthesis of cystathionine requires pyridoxal 5-phosphate (vitamin B6). Although it has been shown that deficiencies of vitamin B12 and folate are related to increased plasma Hcy concentrations[32-35], the relationship of Hcy levels to vitamin B6 status is less clear[36,37]. In addition, excess dietary methionine in normal mice has been shown to induce HHcy[38]. Under normal conditions, several methylation reactions in the liver contribute to the bulk (90%) of SAM utilization and Hcy production via SAH. For example, phosphatidylethanolamine to phosphatidylcholine is mediated by phosphatidylethanolamine N-methyltransferase (PEMT). PEMT-/- mice had 50% decreased plasma Hcy despite being choline and betaine deficient[39]. PEMT null mice exhibited fatty liver and apoptosis but this was not prevented by betaine administration, impaired lipoprotein secretion rather than methyl donor deficiency appeared to be the dominant effect of choline deficiency[40]. The other major source of Hcy is the activity of hepatic guanidinoacetate (GAA) N-methyltransferase (NMT). GAA is produced in the kidney by L-arginine:glycine amidinotransferase. GAA is then converted to creatine in the liver by GAA-NMT, utilizing SAM and generating SAH. Creatine is exported to muscle and also represses the kidney enzyme which produces GAA. GAA supplementation could induce HHcy and creatine feeding lowers Hcy[41].
HCY TOXICITY
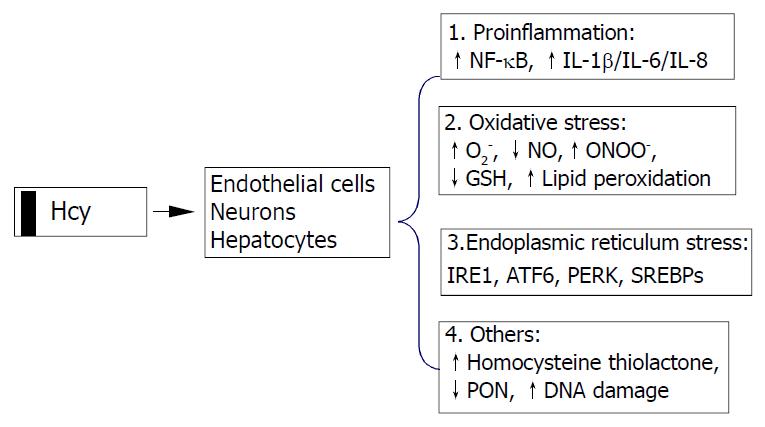
Possible cellular mechanisms by which elevated Hcy promotes liver disease are oxidative stress, endoplasmic reticulum (ER) stress and the activation of pro-inflammatory factors (Figure 2). Hcy enhances the production of several pro-inflammatory cytokines. Expression of monocyte chemoattractant protein 1 (MCP-1) was increased in cultured human vascular endothelial cells, smooth muscle cells and monocytes treated with Hcy[42-44]. Hcy has also been shown to increase expression of IL-8[42], a T-lymphocyte and neutrophil chemoattractant, in cultured endothelial cells. Hcy-induced expression of MCP-1 and IL-8 in monocytes and endothelial cells has been shown to occur through activation of NF-κB, a transcription factor involved in mediating downstream inflammatory processes[44,45]. Active NF-κB could stimulate production of cytokines, chemokines, interferons, leukocyte adhesion molecules, hemopoietic growth factors and major histocompatibility (MHC) class I molecules-all of which are thought to influence inflammation[45,46].
Figure 2 Cellular mechanisms by which homocysteine pro-motes cell injury.
Homocysteine causes activation of necrosis factor-κB (NF-κB) and enhances production of cytokines (IL-1β, IL-6, and IL-8) resulting in inflammatory reactions, increases intracellular levels of superoxide anion causing oxidative stress, and induces endoplasmic reticulum (ER) stress by causing misfolding of proteins traversing the ER. Homocysteinyl-tRNA increases production of highly reactive derivative homocys-teine thiolactone which damages enzymes and DNA. IRE1, type 1 ER transmembrane protein kinase; ATF6, the activating tran-scription factor 6; PERK, the PKR like ER kinase; SREBP, sterol regulatory element binding protein, PON, paraoxonase.
Hcy can generate a procoagulant state, which may be related to its proclivity to auto-oxidize, generating H2O2. Various in vitro studies using vascular tissues have implicated Hcy in causing abnormal vascular relaxation responses by enhancing the intracellular production of superoxide anion (O2-)[47-54]. O2- is believed to react with and decrease the availability of endothelial nitric oxide (NO) and yield peroxynitrite, thereby limiting normal vasodilation responses[55,56]. Deceased GSH peroxidase transcription (reduction of peroxides protects NO) may play a role in this process[49,57], since overexpression of GSH peroxidase could restore the NO response[57]. O2- and peroxynitrite are also known to contribute to the oxidative modification of tissues, resulting in the formation of lipid peroxides and nitrosated end products such as 3-nitrotyrosine. The observations that Hcy decreased the expression of a wide range of antioxidant enzymes[57-59] and impaird endothelial NO bioavailability by inhibiting glutathione peroxidase activity raise the possibility that Hcy sensitizes cells to the cytotoxic effects of agents or conditions known to generate ROS. Decreased NO bioavailability has also been shown in vitro to increase the expression of MCP-1, which may enhance intravascular monocyte recruitment and lead to accelerated lesion formation[60].
Intracellular Hcy can be converted by methionyl tRNA synthase into an Hcy-AMP complex, which is subsequently catabolised to Hcy thiolactone, thereby preventing the incorporation of Hcy into nascent polypeptide chains. Hcy thiolactone has unique reactive properties that can lead to the homocysteinylation of lysine residues and free amine groups on numerous cellular proteins, thereby resulting in decreased biological activity and premature degradation[61]. In addition, Hcy thiolactone secreted into the circulation may induce widespread modifications of plasma proteins that could potentially contribute to the development of liver and cardiovascular diseases. Recent studies have demonstrated that Hcy thiolactone decreases paraoxonase activity associated with HDL, thereby rendering HDL less protective against oxidative damage or against toxicity of Hcy thiolactone[62].
HCY-INDUCED ER STRESS
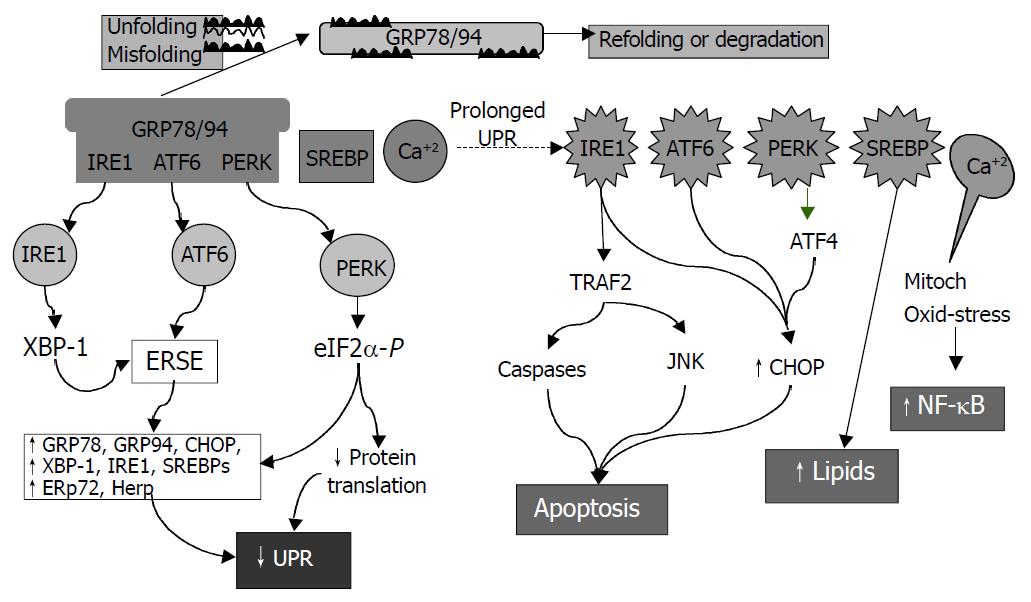
ER is a principal site for protein synthesis and folding, calcium storage and calcium signaling. It also serves as a site of biosynthesis for steroids, cholesterol and other lipids. The physiological roles of the ER include regulation of protein synthesis, folding and targeting and maintenance of cellular calcium homeostasis. The ER has a high concentration of numerous resident chaperone proteins such as glucose regulated protein-78 (GRP78) and GRP94, a high level of calcium and an oxidative environment to carry out these functions efficiently. Proteins that were translocated into the ER lumen underwent post-translational modifications and the folding required for optimal function. Properly folded proteins were allowed to reach their destiny via the secretory pathway, whereas unfolded and misfolded proteins were exported or dislocated from the ER and degraded by cytoplasmic proteasomes[63-68]. ER stress is a condition under which unfolded and misfolded proteins accumulate (Figure 3). ER stress triggers unfolded protein response (UPR), which is an intracellular signaling pathway and is mediated via three ER-resident sensors in mammalian cells: a type-I ER transmembrane protein kinase (IRE-1), the activating transcription factor 6 (ATF-6) and the PKR like ER kinase (PERK). Activation of these three pathways is mediated by GRP78, which is associated with each sensor in the absence of ER stress. As unfolded proteins accumulated in the ER, GRP78 dissociated from and thereby activating IRE-1, ATF-6 and PERK[68-70]. Activation of both IRE-1 and ATF-6 increases the expression of ER-resident chaperones. IRE-1 is a stress-activated transmembrane protein kinase having endoribonuclease activity. Following ER stress, IRE-1 dimerized and was autophosphorylated, thereby allowing IRE-1 to act as an endoribonuclease in the alternative splicing of XBP-1 mRNA. The removal of a 26 base pair intron resulted in a translation frameshift that permits XBP-1 to act as a transcriptional activator of genes containing upstream ER stress response elements (ERSE). Upon ER stress, ATF-6 was transported to the Golgi where the cytosolic transactivation domain of ATF-6 is cleaved from the membrane by specific proteases (S1P and S2P) that also recognize, cleave and activate sterol regulatory element-binding proteins (SREBPs) leading to increased lipids needed for ER membrane synthesis. Following release, the transactivation domain of ATF-6 localized to the nucleus where it interacts with ERSE, thereby activating transcription of numerous UPR-responsive genes, including GRP78, GADD153 (CHOP), XBP-1, ERp72, and Hcy-induced ER protein (Herp). ER stress could also lead to a rapid attenuation in protein synthesis, a cellular process mediated by the transmembrane protein kinase, PERK. Activation of PERK could cause phosphorylation of eukaryotic initiation factor-2α (eIF-2α), which blocks mRNA translation initiation to help relieve the unfolded protein burden on the ER. Recent studies have also demonstrated that PERK-dependent eIF-2 α phosphorylation is required for transcriptional activation of a wide range of UPR-responsive genes[71,72]. The early UPR co-coordinately enhances cell survival by ensuring that the adverse effects of ER stress are dealt with in a timely and efficient manner. However, prolonged UPR following ER stress has severe consequences. It can lead to activation of the tumor necrosis factor receptor associated facter 2 (TRAF2), which activates caspases (e.g. caspase-12 in mice) and JNK resulting in programmed cell death. Over-expression of CHOP, a basic region leucine zipper transcription factor, could also promote cell death[71]. Overproduction of lipids by SREBP can lead to fat accumulation. In addition, ER stress is associated with release of ER Ca2+ stores which can trigger oxidative stress via effects on mitochondria and NF-κB activation leading to inflammatory reactions[73]. NF-κB activation could be blocked by calcium chelators and antioxidants[19]. Increased cytosol calcium also activates calpains which proteolytically cleave Bcl-XL (inactivation) and caspase 12 (activation). ER stress could contribute to the pathogenesis of a number of human diseases, including diabetes, Alzheimer’s disease, Parkinson’s disease and cancer[72].
Figure 3 Consequences of endoplasmic reticulum (ER) stress response.
In the early phase, unfolded proteins cause dissociation of chaperones such as Bip/GRP78 from ER resident kinases-IRE1 and PERK and transcription factor-ATF6. Activated PERK phos-phorylates eIF2 resulting in translational attenuation. Activated IRE1 and ATF6 up-regulate genes encoding ER chaperone pro-teins such as GRP78/94 leading to increased protein-folding capacity. Overall, the unfolded protein response (UPR) goes down. In the late phase, IRE1 interacts with TRAF2 (tumor necrosis factor receptor associated factor 2) which activates caspases and JNK (cJUN NH2-terminal kinase) leads to apoptosis. ATF6 and PERK upregulate CHOP (C/EBP homologous protein) promoting cell death. SREBP upregulates lipid synthesis. Prolonged UPR leads to Ca2+ release from ER causing production of reactive oxygen intermediates which may lead to activation of NF-κB.
Hcy induced ER stress response has recently received much attention[6,74-79]. Hcy causes ER stress by disrupting disulphide bond formation and causing misfolding of proteins traversing the ER. Elevated levels of intracellular Hcy could increase the expression of several ER stress response genes, including GRP78, GRP94, Herp and RTP[6,58,74,77,78,80-82]. Hcy could induce expression of GADD153[58,78,79] involved in ER stress-induced cell death[83]. Hcy-induced ER stress could cause dysregulation of lipid biosynthesis by activating the SREBPs[6,76-79], ER resident transcription factors are responsible for the induction of genes in the cholesterol/triglyceride biosynthesis and uptake pathways[6]. Hcy-induced cell death was mimicked by other ER stress agents and was dependent on IRE-1 signaling. Activation of IRE-1 by Hcy could lead to a rapid and sustained activation of JNK protein kinases[84,85], a result consistent with the finding that activation of JNK by ER stress involved binding of IRE-1 to TRAF2[86]. Because persistent activation of JNK correlated with cell death[87], these studies could provide further support for a mechanism involving Hcy-induced programmed cell death.
APPROACHES FOR STUDY OF HHCY
Cell and animal models with altered plasma Hcy are among the most useful approaches in determining the biological effects of HHcy. However, cell and transgenic animal models expressing Hcy metabolism-related genes/enzymes are not available. Nevertheless, diet- and, especially, genetic-induced animal models of HHcy have been developed. The gene knockout animals have significantly enhanced the status of Hcy as an independent risk factor for several disorders.
Homozygous and heterozygous CBS-deficient mice were generated in 1995[88]. Homozygous mutants completely lacking CBS were born at the expected frequency from mating of heterozygotes, but they suffered from severe growth retardation and a majority of them died within 5 wk after birth. Histological examination showed that the hepatocytes of homozygotes were enlarged, multinucleated, and filled with microvesicular lipid droplets. Plasma Hcy levels of the homozygotes (203.6 ± 65.3 μmol/L) were 33 times higher than normal (6.1 ± 0.8 μmol/L). The homozygous CBS deficient mice represented a model for severe HHcy. Heterozygous CBS deficient mice had 50% reduction in CBS mRNA and enzyme activity in the liver and had twice normal plasma Hcy levels (13.5 ± 3.2 μmol/L). The CBS knockouts significantly help elucidate the in vivo role of elevated levels of Hcy in the etiology of several HHcy-related disorders and in the cellular mechanisms by which Hcy promote cell injury. The CBS-deficient mice were predisposed to HHcy during dietary folate deficiency, and moderate HHcy was associated with marked impairment of endothelial function in mice[89]. Results from a subsequent study indicated that endothelial dysfunction occurred in HHcy mice even in the absence of folate deficiency[90]. Endothelial dysfunction in CBS (+/-) mice was associated with increased tissue levels of SAH, which suggests that altered SAM-dependent methylation may contribute to vascular dysfunction in HHcy[91]. Further studies with the CBS deficient mice revealed the importance of intracellular redox balance for nitric oxide bioactivity and endothelial function, and the importance of ER stress in abnormal hepatic accumulation of lipid[49,92]. Expression of several genes analyzed by DNA microarray was found to be reproducibly abnormal in the livers of heterozygous and homozygous CBS-deficient mice[93]. These genes encode ribosomal protein S3a and methylthioadenosine phosphorylase, suggesting cellular growth and proliferation perturbations may occur in homozygous CBS-deficient mice liver.
MTHFR-deficient mice have been recently developed to examine the effects of HHcy resulting from genetic deficiencies in the remethylation pathway[94]. MTHFR-deficient mice shared basic phenotypic similarities with CBS-deficient mice. However, they were unique in that they developed mild HHcy and atherosclerosis. Recent study has demonstrated the importance of choline metabolism in HHcy in this model[95]. Comparison study by administrating the alternate choline-derived methyl donor, betaine, to wild-type mice and MTHFR deficient mice revealed that plasma Hcy and liver choline metabolite levels were strongly dependent on the MTHFR genotype. Betaine supplementation decreased Hcy in all three genotypes, restored liver betaine and phosphocholine pools, and prevented severe steatosis in MTHFR-deficient mice. Since there was a significant negative correlation between plasma betaine and Hcy concentrations in humans with cardiovascular disease, the results emphasize the strong interrelationship between Hcy, folate, and choline metabolism. MTHFR-compromised mice with HHcy appeared to be much more sensitive to changes of choline/betaine intake than wild-type animals. HHcy, in the range of that associated with folate deficiency or with homozygosity for the 677T MTHFR variant, may be associated with disturbed choline metabolism.
MS could directly catalyze the remethylation pathway and inactivation of this gene has been attempted recently[96]. Heterozygous MS knockout mice from an outbred background had slightly elevated plasma Hcy (6.1 mol/L) and methionine compared to wild-type mice (4.1 μmol/L) but seemed to be otherwise indistinguishable. Homozygous knockout embryos survived through implantation but died soon thereafter. Nutritional supplementation during pregnancy was unable to rescue embryos that were completely deficient in MS. This study indicated that MS activity was essential for early embryonic development of mice. Although the MS knockout mouse has not provided an immediately obvious animal model of human disease, heterozygotes with 50% reduction of MS activity may be useful. It is likely that MS heterozygote knockouts are more susceptible to dietary deficiencies than wild type mice and thus having merits as a model in which interactions between genetic status and nutritional status can be studied.
The animal models are valuable in vivo tools to further examine potential therapeutic approaches in lowering plasma Hcy while decreasing the prevalence of HHcy-induced disorders. However, the animal models neither have tissue or organ specificity nor exclude potential compensatory pathways of Hcy metabolism. Conditional disruption of Hcy metabolism-related genes and crossing between animal models that are deficient in different genes should be the future directions in the effort of creating animal models for study of HHcy.
ETHANOL-INDUCED HHCY AND LIVER INJURY
The pathogenesis of the pathologic features of alcoholic liver injury, namely steatosis, apoptosis, necrosis, inflammation and fibrosis, is an area of intense interest. Although much progress has been made over the past decade, we still do not have a complete understanding of this process[97]. We recently found that in a murine model of intragastric ethanol there was an upregulation of genes associated with endoplasmic reticulum (ER) stress response, including GRP78 and 94, CHOP and SREBP. The expression of these genes was associated with protein malfolding as well as apoptosis and lipid synthesis[78,79]. Since alcoholism and alcohol-related diseases constitute a severe health problem in the world and ER stress has been linked to Hcy in the pathogenesis of several disorders such as atherosclerosis, Alzheimer’s disease, and liver steatosis, we would direct the readers’ attention to ethanol-induced alterations in Hcy metabolism.
Alcoholic patients have been shown to have elevated plasma Hcy (average two-fold) which rangeed from 10-120 mol/L (normal 5-15 mol/L)[98-100]. Total folate, B12 and B6 levels were normal. However, Hcy levels correlated with folate levels and blood alcohol levels. Well nourished alcoholics exhibited markedly lower levels of serum pyridoxal-phosphate (PLP) and mildly lower red cell folate[37]. Even “social” drinking (30 g/d×6 wk) caused 20% increased Hcy and decreased folate[98,101]. Heavy alcohol consumption is a risk factor for stroke and brain atrophy[101-103]. Rats fed ethanol exhibited a doubling of plasma Hcy despite normal levels of folate, PLP and B12[100]. We have observed a 7 fold increase of plasma Hcy levels (22.3 ± 2.8 μmol/L vs pair-fed control 3.0 ± 0.9 μmol/L) in mice fed ethanol intragastrically for 4 wk[78].
With alcohol feeding of rats intragastrically for 9 wk liver specific MAT1A protein expression did not change, whereas MAT2A increased in conjunction with -40% decreased hepatic levels of methionine and SAM[104]. However, shorter exposure of rats and minipigs to ethanol was not associated with a decrease in methionine or SAM in most studies. Depending on route, ethanol dose and duration, variable changes in SAM and SAH have been described[104-107].
Ethanol feeding has been known to lower MS[108], leading to increased accumulation of 5-methyl THF and to increased BHMT leading to utilization-induced decreased betaine levels[108]. These effects depended on increased blood ethanol. Golden Syrian hamsters with high ADH fed a 360 mL/L ethanol diet did not develop increased blood ethanol levels or changes in Hcy metabolism unless ADH was inhibited[109]. Of note, the SAM levels were maintained by the utilization of betaine. However prolonged ethanol feeding eventually could lead to depletion of SAM. Chronic alcohol could increase choline uptake[110] and mitochondrial oxidation to betaine[111] suggesting compensation for increased demand for betaine. Feeding betaine (0.5%) raised SAM levels which was accentuated in alcohol fed animals (minimal to begin with) and prevented fatty liver[78,105,108]. Raised SAM was initially assumed to contribute to betaine’s ameliorative effect on fatty liver. It may be equally important that the protective role of betaine was due to lowering Hcy directly through BHMT and indirectly by raised SAM leading to activation of CBS.
The mechanism of the ethanol induced decrease in MS is not well understood. Kenyon et al[107] showed that the enzyme was inhibited by high concentrations of acetaldehyde, whereas we have found decreased mRNA. Increase in BHMT activity appeared to be a compensatory phenomenon to maintain methionine and was seen after 2 wk in ethanol fed rats and after 4 wk in ethanol fed mice.
In the chronic (12 mo) ethanol fed micropigs with adequate folate, MS activity decreased by 20% which was associated with slightly decreased serum methionine, 20% increased serum Hcy, and increased hepatic SAH but no change in SAM. These small changes due to ethanol were not associated with increased ALT or fatty liver but were associated with increased scattered apoptosis[112]. Interestingly addition of folate deficient diet to ethanol feeding of the castrated minipig accentuated plasma Hcy and liver injury[116] although ER stress was not considered in this study.
Betaine lowered Hcy and prevented ER stress and alcoholic liver injury in alcohol fed mice[78]. However, we need to be cognizant of other actions of betaine. Feeding rats betaine in drinking water (1.5 g/kg) blunted the TNF response to LPS and decreased concomitant liver injury[114]. Importantly, however, taurine was equally effective. Earlier work has suggested an indirect protective role of choline supplementation, suggesting choline oxidation to betaine could protect against Kupffer cell activation[115-117]. It has been suggested that betaine and taurine serve as organic osmolytes which are critical in regulating Kupffer cell function[118]. Hyperosmotic conditions induce Na+ betaine transporter mRNA while hypoosmotic conditions do the opposite, this occurred in Kupffer cells but not hepatocytes. Betaine or taurine protects the liver against warm ischemia-reperfusion. Recently, betaine pretreatment was shown to protect the hepatocyte from bile acid induced apoptosis. The mechanism is not certain and high concentrations of betaine (mmol/L) were required[119,120]. We observed that increased gene expression of TNF and CD 14 was indicative of the alcohol-induced Kupffer cell activation[78]. However, betaine treatment did not significantly attenuate these changes, suggesting that betaine either acts downstream of alcohol-induced Kupffer cell activation or acts via an independent pathway.
The effect of SAM feeding is of interest since it was reported to decrease fatty liver and mitochondrial abnormalities[112]. SAM might be expected to inhibit re-methylation and promote transsulfuration of Hcy. It is unclear as to what the overall effect on Hcy would be. Severe SAM deficiency in MAT1A knockout did not alter Hcy but was associated with increased expression of BHMT and CBS[121]. SAM could transcriptionally activate MAT1A and suppress MAT2A[122].
Overall chronic ethanol exposure seemed to cause a modest decrease in SAM and increase in SAH along with early-decreased MS and late-increased BHMT. All these changes were accompanied with increased Hcy which occurs despite adequate dietary folate, B12, B6 and choline. Thus there are possible contributions of decreased MS, unknown effects on CBS, and decreased SAM (decreased activation of CBS) leading to HHcy. The decrease in SAM levels was accompanied with increased SAH levels. Since both SAM and SAH activated CBS, it is doubtful that changes in levels of these metabolites exerted a significant regulatory role on transulfuration[11,16]. The increase in BHMT appeared insufficient to lower Hcy due to limitation on the availability of betaine and already-impaired cell function. Although high dietary choline might generate sufficient betaine in rodents, the choline oxidase pathway is normally low in primates. Thus, providing excess dietary betaine rather than choline would seem to be an approach more applicable to the human situation. Since betaine corrects hyperhomocysteinemia, fatty liver injury and ER stress, and homocysteine is known to cause all these changes, it is reasonable to state that an important mechanism by which betaine protects against alcoholic liver disease is the correction of hyperhomocysteinemia and proof of this hypothesis requires further work.
POSSIBLE ROLE OF ER STRESS IN OTHER LIVER DISEASES
ER stress may also be involved in liver injury caused by α1-antitrypsin (α1-AT) deficiency and hepatitis C virus (HCV) or hepatitis B virus (HBV) infection. α1-antitrypsin (α1-AT) deficiency was caused by a point mutation encoding a substitution of lysine for glutamate-342[123]. Aggregated mutant α1-AT was retained in ER rather than secreted in the blood and body fluids where its function is to inhibit neutrophil proteases. Individuals with this deficiency had a markedly increased risk of developing emphysema by a loss of function mechanism by which reduced levels of α1-AT in the lung inhibit connective tissue breakdown by neutrophil elastase, cathepsin G, and proteinase 3. Some individuals with α1-AT deficiency developed liver injury and hepatocellular carcinoma by a gain of function mechanism, i.e., accumulation of aggregated mutant α1-AT within the ER which is toxic to liver cells. However, the exact mechanism by which ER retention of this aggregated mutant protein leads to cellular injury is still unknown. Recent studies have demonstrated that ER retention of mutant α1-AT induces a marked autophagic response in cell culture and transgenic mouse models of α1-AT deficiency as well as in the liver of patients with α1-AT deficiency[124]. The autophagic response is a general mechanism whereby cytosol and intracellular organelles, such as ER, are first sequestered from the rest of the cytoplasm within unique vacuoles and then degraded by fusion with lysosomes to clear the cells of senescent constituents. Under a fasting condition, a marked increase in fat accumulation was observed in α1-AT-containing globules in the liver of α1-AT deficient mice[125], suggesting a malfunction of ER caused by accumulation of mutant α1-AT. Investigations of ER stress markers such as GRP78, CHOP, SREBP, XBP1, and ATF6 are needed to assess the direct involvement of ER stress in α1-AT deficiency-induced liver injury.
Evidence of ER stress in HBV or HCV infection is emerging. HBV codes for three forms of surface protein. The minor and large forms are translated from transcripts specified by the preS1 promoter, while the middle and small forms are translated from transcripts specified by the downstream S promoter. Overexpression of the large surface protein of HBV could lead to a 10-fold activation of the S promoter but not of an unrelated promoter[126]. The large surface protein could also activate the cellular grp78 and grp94 promoters. Neither the middle nor the small surface protein, nor a secretable form of the large surface protein, could activate the S promoter, but agents that induced endoplasmic reticulum (ER) stress had an effect similar to that of the large surface protein, suggesting that HBV may evolve a feedback mechanism, such that ER stress induced by accumulation of the large surface protein increases the synthesis of the middle and small surface proteins, which in combination with the large surface protein can form mixed, secretable particles. HCV-induced ER stress was more evident. HCV replicates from a ribonucleoprotein (RNP) complex that is associated with ER membrane. The replication activities of the HCV subgenomic replicon have been shown to induce ER stress[127]. HCV replicons induce the UPR which is paralleled by the proteolytic cleavage of ATF6. The HCV non-structural protein 5A (NS5A) can bind to and inactivate the cellular double-stranded RNA-activated protein kinase, PKR. NS5A has recently been demonstrated to engage ER-nucleus signal transduction pathway[131]. Expression of NS5A in the ER could induce an ER stress leading to the activation of STAT-3 and NF-κB, which is sensitive to inhibitors of Ca2+ uptake. The NS5A-induced ER stress signaling has also been shown in the context of an HCV subgenomic replicon[128]. Another HCV component, the HCV envelope protein E2, is an ER-bound protein that contains a region of sequence homology with the PKR and its substrate, the eIF2α. E2 could modulate global translation by inhibition of the interferon-induced PKR through its PKR-eIF2α phosphorylation site homology domain (PePHD)[129]. E2 could also bind to and inhibit PERK[129]. At low expression levels, E2 induced ER stress, but at high expression levels, E2 inhibited PERK kinase activity in vitro. Mammalian cells that stably expressed E2 were refractory to the translation-inhibitory effects of ER stress inducers, and E2 relieved general translation inhibition induced by PERK. The PePHD of E2 was required for the rescue of translation that was inhibited by activated PERK. These findings may explain why the virus promotes persistent infection by overcoming the cellular ER stress response. In addition, HCV-induced ER stress resulted in a decline in protein glycosylation. Decreasing protein glycosylation could disrupt the proper protein folding of MHC class I molecules, preventing the assembly of MHC class I molecules. Cells expressing HCV subgenomic replicons had a lower MHC class I cell surface expression[130]. HCV-infected cells may thus be undetectable in the immune system by suppressing MHC class I antigen presentation to cytotoxic T lymphocytes. Therefore, the persistence and pathogenesis of HCV may depend upon the ER stress-mediated interference of MHC class I assembly and cell surface expression. Finally, HCV infection may suppress the degradation of misfolded proteins while stimulating the synthesis of its viral proteins in the ER. In the ER, IRE1-XBP1 pathway directs both protein refolding and degradation in response to ER stress. It was demonstrated that XBP1 expression was elevated in cells carrying HCV subgenomic replicons, but XBP1 transactivating activity was repressed[131]. This prevents the IRE1-XBP1 transcriptional induction of EDEM (ER degradation-enhancing -mannosidase-like protein), which is required for the degradation of misfolded proteins. Consequently, misfolded proteins are stable in cells expressing HCV replicons. Study with a cell line with a defective IRE1-XBP1 pathway showed elevated levels of HCV internal ribosome entry site-mediated translation[131]. This study indicated that the HCV suppression of misfolded protein degradation in the ER not only promoted HCV expression but also contributed to the persistence of the virus in infected hepatocytes.
HCV infection is common in alcoholic patients presenting with liver disease. Heavy alcohol intake would worsen the outcome of HCV infection[132-134], which has directed much attention to the interaction between alcohol and HCV infection. Alcohol plays an important role in HCV infection resulting in increased viral replication, enhanced HCV quasispecies complexity, increased liver-cell death, suppression of immune responses, and iron overload[135]. However, the pathogenic mechanisms underlying the alcohol-HCV interactions are not fully understood. Based on the above evidence that both HCV and homocysteine could induce endoplasmic reticulum (ER) stress response[6,78,127] and that there was a link between alcohol-induced significant elevation of homocysteine level, ER stress, and the pathogenesis of liver injury[78], it is reasonable to hypothesize that a locus of the potentiative interaction between alcohol and HCV in accelerating the progression of liver disease is at the level of ER stress. In the case of both HBV and HCV infection, it is widely recognized that severely immunosuppressed patients may develop a paradoxically severe and rapidly progressive liver disease. This has been seen in the post-OLT setting and in patient with AIDS. Therefore, the loss of immune detection of viral-infected hepatocytes may lead to an unopposed massive overload of hepatocytes with viral proteins triggering ER stress. Future studies should examine the contribution of ER stress to these pathologic conditions.
CONCLUSION
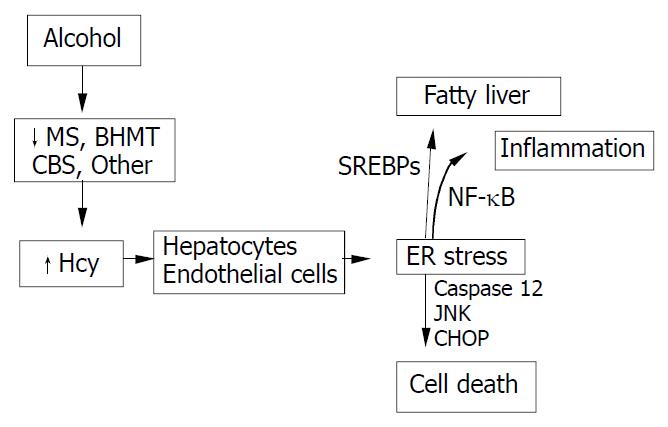
HHcy is an integral component of several disorders including cardiovascular and cerebrovascular diseases, neurodegeneration, liver steatosis, diabetes, and cancer. HHcy can result from deficiencies of vitamin cofactors (B6, B12, folic acid) required for Hcy metabolism and/or from genetic disorders of its metabolism. Hcy unleashes inflammation mediators such as NFκB, IL-1β, IL-6, and IL-8. Hcy increases production of intracellular superoxide anion causing oxidative stress. Hcy-induced misfolding or malfunctioning of numerous intracellular proteins are increasingly important and attract much attention because the Hcy-induced ER stress mechanism can explain many processes of cell injury. Animal model creation and integral investigation of available animal models will certainly play important role in determining precisely the biological effects of HHcy. Our observations with the murine intragastric ethanol fed model have suggested a link between Hcy metabolism, ER stress, and the pathogenesis of alcohol induced liver injury. Figure 4 demonstrates our hypothesis in which ethanol feeding causes HHcy which then induces the ER stress response in parenchymal and nonparenchymal cells in the liver leading to fatty liver, apoptosis and possibly inflammation. The potential beneficial effects of lowering Hcy and preventing ER stress in alcoholic humans needs to be studied. In addition, since a minority of alcoholics develop liver disease and a wide range of Hcy levels are seen in alcoholics, it will be important to examine polymorphism of Hcy metabolizing enzymes as potential risk-factors for the development of HHcy and liver disease.
Figure 4 Hypothesis for the role of ethanol-induced HHcy in the pathogenesis of alcoholic liver disease.