Published online Jun 1, 2004. doi: 10.3748/wjg.v10.i11.1682
Revised: September 4, 2003
Accepted: September 18, 2003
Published online: June 1, 2004
AIM: To study the expression of CDw75 in patients with gastric carcinoma and to correlate CDw75 expression with progression of the tumor.
METHODS: Immunohistochemical method was used to examine the expression of CDw75 in 72 cases of the gastric carcinoma and adjacent normal gastric mucosa, and the percentage of the cells positively stained with CDw75 was calculated using a computer-aided microscopic image analysis system.
RESULTS: CDw75 was not expressed in normal gastric mucosa but detected in 37 of the 72 neoplastic gastric lesions. The expression of CDw75 was associated with the tumor progression as indicated by its close correlation with the depth of the tumor infiltration (χ2 = 18.415, P < 0.01), TNM stage (χ2 = 10.419, P < 0.05) and lymph node metastasis (χ2 = 6.675, P < 0.01). The overall survival rate of the patients with positive CDw75 expression (32.4%) was significantly lower than that of the patients without CDw75 expression (71.4%) (P < 0.01). There was no significant correlation between the expression of CDw75 and the sex and age and histological type of patients (P > 0.05).
CONCLUSION: These findings suggest that the expression of CDw75 is a significant histopathological marker for more advanced stage of gastric carcinoma and indicates a poor prognosis for the patients.
- Citation: Shen L, Li HX, Luo HS, Shen ZX, Tan SY, Guo J, Sun J. CDw75 is a significant histopathological marker for gastric carcinoma. World J Gastroenterol 2004; 10(11): 1682-1685
- URL: https://www.wjgnet.com/1007-9327/full/v10/i11/1682.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i11.1682
Invasiveness and metastasis are the most important characteristics of malignant tumor and mortality factors. Clinicopathological parameters are usually used for diagnosis and prognosis. Recent studies have shown that the expression of sialylated glycoconjugates is closely associated with the aggressiveness and metastatic potential of malignant cells. CDw75 epitope is a sialylated carbohydrate determinant generated by the β-galactosyl α-2,6-sialyltransferase, and it has been reported to be associated with the progression of gastric cancer[1]. Gastric cancer is one of the most common tumors of alimentary tract in China, and its mortality is high. In this study, we examined the expression of CDw75 in 72 cases of gastric carcinoma and adjacent normal gastric mucosa using immunohistochemical method to determine whether CDw75 is correlated with the invasiveness and metastasis of gastric carcinoma.
Seventy-two patients with gastric cancer, who were diagnosed and treated at the Departments of Pathology and General Surgery, Renmin Hospital of Wuhan University, from 1995 to 1999, were randomly selected for this study. The patients had undergone subtotal or total gastrectomy combined with lymph node resection. Sections from the surgical specimens were fixed in 40 g/L formaldehyde and embedded in paraffin. The mean age of the patients was 59 years with a range from 35 to 74 years and a male-to-female ratio of 1.48. The patients were followed to determine clinical outcome and the median follow-up time at the end of the study was 45 mo (range, 3-64 mo). The other clinicopathological characteristics are listed in Table 1. The histological type of the tumor was defined by World Health Organization classification (tubular, papillary, mucinous, or signet ring[2], the degree of differentiation (well, moderate, or poor) was recorded on haematoxylin and eosin (H & E) stained tissues, and tumor stage was graded according to new PTNM criterion which was published by International Union Against Cancer (IUCC)[2].
| Expressing levels of CDw75 | P | ||||
| Negative Positive Positive rate (%) | |||||
| Sex | |||||
| Male | 43 | 24 | 19 | 44.19 | 2.217 |
| Female | 29 | 11 | 18 | 62.07 | |
| Age (yr) | |||||
| < 50 | 17 | 9 | 8 | 47.06 | 0.167 |
| ≥ 50 | 55 | 26 | 29 | 52.73 | |
| Histologic type | |||||
| WD | 12 | 8 | 4 | 33.33 | 3.445 |
| MD | 16 | 9 | 7 | 43.75 | |
| PD | 35 | 15 | 20 | 57.14 | |
| Mucinous | 5 | 2 | 3 | 60.00 | |
| Signet ring | 4 | 1 | 3 | 75.00 | |
| Depth of invasion | |||||
| T1 | 8 | 7 | 1 | 12.50 | 18.415b |
| T2 | 17 | 13 | 4 | 23.53 | |
| T3 | 32 | 13 | 19 | 59.38 | |
| T4 | 15 | 2 | 13 | 86.67 | |
| TNM stage | |||||
| I | 24 | 17 | 7 | 29.17 | 10.419a |
| II | 8 | 5 | 3 | 37.50 | |
| III | 20 | 8 | 12 | 60.00 | |
| IV | 20 | 5 | 15 | 75.00 | |
| Lymph node metastasis | |||||
| Negative | 32 | 21 | 11 | 34.38 | 6.675b |
| Positive | 40 | 14 | 26 | 65.00 | |
| Distant metastasis | |||||
| M0 | 56 | 30 | 26 | 46.43 | 2.482 |
| M1 | 16 | 5 | 11 | 68.75 | |
Streptavidin-peroxidase (S-P) method was used to detect the expression of CDw75. The mouse monoclonal antibody against human CDw75 (LN1) was purchased from NeoMarkers Company, Wuhan, China, immunostaining S-P kit and DAB reagent were purchased from Fuzhou Maxim Biotechnical Company, Fuzhou, China. Sections from each primary tumor and adjacent mucosa were deparaffinized and heated in a microwave oven for 15 min to retrieve antigens. Endogenous peroxidase was blocked with 3 mL/L hydrogen peroxide methanol for 10 min at room temperature. After washing with phosphate-buffered saline (0.01 g/L, pH7.4) for 3 × 5 min, the tumor sections were incubated with normal non-immune serum from bull for 15 min at room temperature to eliminate nonspecific staining. The sections were then incubated with the primary antibody against CDw75 (dilution 1/100) for 60 min at room temperature, washed with PBS for 3 × 5 min, and incubated with the secondary biotinylated antibody for 15 min followed by avidin-biotin-peroxidase for 15 min at room temperature. Finally, the slides were washed for 3 × 15 min with PBS, visualized with DAB reagent and counterstained with haematoxylin. Negative and positive controls were used simultaneously to ensure specificity and reliability of the staining process. The negative controls were performed by substituting the primary antibody with PBS, and a positive section supplied by manufacturer of the staining kit was taken as positive control.
Positive staining with CDw75 was defined as brown staining of cell membrane or cytoplasm. The degree of the CDw75 staining was calculated semiquantitatively with computer-aided analysis of four non-overlapping high power microscopic fields and classified as follows: –, negative staining; +, less than 25% tumor cells were CDw75 positive; ++, between 25% and 50% of tumor cells were CDw75 positive; +++, between 51% and 75% of tumor cells were CDw75 positive; and ++++, more than 75% of tumor cells were CDw75 positive.
CDw75 expression in all patients with gastric carcinoma was analyzed against clinicopathological parameters. The correlation between CDw75 expression and selected clinicopathological parameters was analyzed with the Wilcoxon rank sum test and χ2 test. Kaplam-meier curve was constructed to assess the survival. P value of less than 0.05 was considered to be statistically significant.
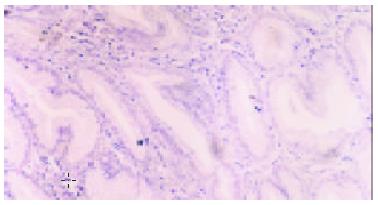
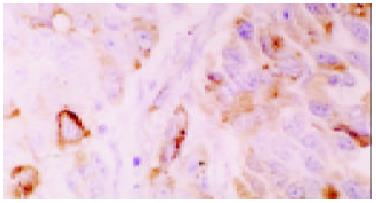
Normal gastric mucosa was consistently negative for CDw75 staining (Figure 1), while positive staining was observed in 37 of 72 (51.4%) gastric cancers. The staining intensity was as follows: -, 35 cases (48.6%) ; +, 9 cases (12.5%) ; ++,13 cases (18.1%) ; +++, 9 cases (12.5%) ; ++++, 6 cases (8.3%). The positive staining of CDw75 was localized predominantly in gastric cancer cells, and all the positive cases showed diffuse membrane staining, or cytoplasmic staining in some cases (Figure 2). The immunoreactive cells were either distributed unevenly throughout the tumor tissue or aggregated in focal clusters. In addition, positive staining was occasionally detected in lymphocytes or other inflammatory cells infiltrating the cancer nests (Figure 3).
The CDw75 expression in tumor tissues was related significantly to clinicopathologic factors, such as the depth of tumor invasion, TNM stageand lymph node metastasis (Table 1). The percentage of CDw75 expression in tumors which invaded serosal or deeper layers (T3 or T4) was significantly higher than that in tumors which were restricted within mucosa or muscular layers (T1 or T2) (P < 0.01). When tumors were accompanied by lymph node metastasis, CDw75 expression was elevated significantly compared with those without metastasis (P < 0.05). The cases were also categorized by TNM staging. To compensate the error due to shortage of cases, stage I and stage II cases were combined into one group. CDw75 expression was found in 10 of 32 (31.3%) stage I + II cases, 12 of 20 (60.0%) stage III cases and 15 of 20 (75.0%) stage IV cases. The percentage of positive CDw75 staining in stage III and IV cases was significantly higher than that in stage I + II (P < 0.05 and P < 0.01, respectively), while there was no significant difference in those between stage III and stage IV (P > 0.05).
No significant correlation was found between the expression of CDw75 antigen and the distant metastasis or the histological type of gastric cancer. Similarly, no significant difference was found between the cases with positive and negative CDw75 expression regarding the sex and age of patients.
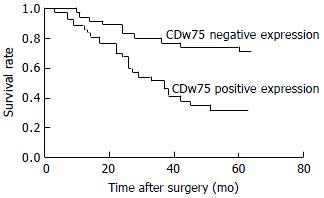
At the end of the follow-up period, 37 of 72 patients (51.4%) were alive. The survival of patients with positive CDw75 expression was significantly shorter than those with negative CDw75 expression (Table 2). AS shown by the Kaplam-Meier curve, the overall survival rate of patients with CDw75 expression (32.4%) was significantly lower than that of patients without CDw75 expression (71.4%) (P < 0.01, Figure 4).
| CDw75 expression | Death/survival | Median survival time |
| Negative (n = 35) | 10/25 | 53 |
| Positive (n = 37) | 25/12 | 37 |
The infiltration and metastasis of gastric carcinoma is a multi-stage, complicated process, which could be influenced by many factors, such as the formation of neoplastic vessels, degradation of tissue membrane and matrix, mobility of tumor cells and quantity and activity of infiltrating leucocyte inside the tumor tissues. Recently, many tumor makers have been identified which are used not only for diagnosis but also for assessment of aggressiveness and prognosis of tumors[3-8]. Tumor markers are antigens or other biological products generated by tumor cells, which are present few or none in normal and non-neoplastic tissues. They usually indicate the change of genes associated with development of tumor and can be detected in tumor tissues or body humour or excretions of the patients[9,10]. CDw75 antigen is one plausible tumor marker that has been shown recently to be associated with the progression and metastasis of gastric carcinoma[1]. Consistent with previous finding[1], the present work demonstrated that CDw75 expression was constantly negative in normal gastric mucosa but positive in 37 of 72 (51.4%) gastric cancers.
CDw75 antigen is a cluster of differentiation antigen of human leucocyte designated at the fourth World Leucocyte-Type Conference in 1989[1]. It is a sialylated carbohydrate determinant generated bySia-T1[11] and has many different isomers, which are specifically expressed in different tissues and can be recognized by corresponding monoclonal antibodies. There have been 4 monoclonal antibodies raised to react with CDw75 antigen, including EBU-141, OKB4, HH2 and LN1, etc.[1]. These CDw75 monoclonal antibodies are likely to identify spatially related structures since binding inhibition studies have shown that binding of each CDw75 monoclonal antibody blocks the binding of the other CDw75 monoclonal antibody. The epitopes recognized by HH2, LN1 and EBU-141 are completely destroyed when cells are treated with neuraminidase, suggesting that sialic acid is a part of these CDw75 determinants[1,12]. In contrast, when the epitope reacts with neuraminidase, the binding of OKB4 with CDw75 epitope is increased, indicating that the OKB4 epitope may be masked by sialic acid and can appear after treatment with the neuraminidase[1,12]. These results suggest that sialic acid may have determinant impact on the character of CDw75 antigen.
Furthermore, studies have shown that cell-surface glycoconjugates play an important role in cell proliferation, adhesion, metastasis and immungenicity. Only the subclone of primary tumor cells that express specific glycoconjugates has metastatic ability[1,12]. The composition and structure of cell surface glycoproteins change frequently during the neoplastic transformation, and most of these changes are the extensive sialyation of cell surface glycoproteins. Moreover, tumor-associated expression of sialylated glycoconjugates has been found to be closely associated with aggressive activity and increased metastatic potential of malignant cells[3]. There are several plausible mechanisms underlining such a correlation: sialic acid reduces the attachment of tumor cells to collagen type IV and fibronectin, masks antigen determinants, inhibits the action of natural killer cells, and induces immunologic tolerance through an increase of the serum half-life of glycoconjugates, etc[1,12]. In gastric carcinoma, various sialylated glycoconjugates, such as Lewis X (Lex), Lewis A (CA-19-9) and sialosyl-Tn, etc, have become clinically important in the detection of aggressive behavior of tumor cells and prediction of disease prognosis[13-16].
CDw75, a Sia-T1-dependent sialylated glycoconjugate, is associated with the biological behaviors of tumors[12,17]. In patients with gastric carcinoma, David and Elpek et al[1] found that there was no CDw75 expression in normal gastric mucosa (except a few isolated parietal cells in the body of the stomach), foveolar hyperplasia, intestinal metaplasia and the adjacent tissues to carcinoma. In contrast, the expression of CDw75 in primary tumor and the metastatic focus of gastric carcinoma was significantly increased. The CDw75 antigen could, therefore, be used as a marker of malignant transformation of gastric epithelium. Our study agreed with their findings. In addition, we found that the expression of CDw75 antigen is closely associated with the depth of the tumor invasion. Patients with serosal or deeper layer invasion of the tumor (T3 or T4) showed significantly higher expression of CDw75 than those with the tumor restricted within mucosa or muscular layers (T1 or T2), indicating that the gastric cancer cells with CDw75 high expression might have increased potential to invade into adjacent tissues. The most important feature of gastric carcinoma is its infiltrative growth and the earliest metastasis path is lymph node metastasis. We found that the positive expression rate of CDw75 (65%) in gastric cancer tissues with lymph node metastasis was significantly higher than that without lymph node metastasis (34%). With regard to TNM stage, CDw75 expression in stage I or stage II tumor tissues was not much higher than normal mucosa, but significantly higher in stage III and stage IV tumor tissues.. These findings suggested that CDw75 plays an important role in the progression of gastric cancer from localized lesion to metastasized neoplasia. As a result, overall survival rate of patients with CDw75 expression (32.4%) was lower than that of patients without CDw75 expression (71.4%), and the survival curve of patients with CDw75 expression was significantly poorer than that of patients without CDw75 expression by Kaplam Meier analysis.. In conclusion, CDw75 was not detected in normal gastric mucosa but expressed in gastric cancer tissues and the higher expression rate was seen in patients with deeper tumor invasion, higher TNM stage and in patients with lymph node metastasis. The survival time of patients with CDw75 expression was less than that of patients without CDw75 expression. Therefore, CDw75 appears to be a useful marker indicating more advanced stage of the malignancy and poor prognosis in patients with gastric carcinoma.
Edited by Liu HX and Xu FM
| 1. | Elpek GO, Gelen T, Karpuzoğlu G, Karpuzoğlu T, Keles N. Clinicopathologic evaluation of CDw75 antigen expression in patients with gastric carcinoma. J Pathol. 2001;193:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Luk GD. Tumors of the stomach In: Mark Feldman, Brwe F. Gastrointestinal and Liver Disease. Volume I, 6th, Edition. Science Press, 2001, Beijing. . |
| 3. | Zheng CX, Zhan WH, Zhao JZ, Zheng D, Wang DP, He YL, Zheng ZQ. The prognostic value of preoperative serum levels of CEA, CA19-9 and CA72-4 in patients with colorectal cancer. World J Gastroenterol. 2001;7:431-434. [PubMed] |
| 4. | Duraker N, Celik AN. The prognostic significance of preoperative serum CA 19-9 in patients with resectable gastric carcinoma: comparison with CEA. J Surg Oncol. 2001;76:266-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Marrelli D, Roviello F, De Stefano A, Farnetani M, Garosi L, Messano A, Pinto E. Prognostic significance of CEA, CA 19-9 and CA 72-4 preoperative serum levels in gastric carcinoma. Oncology. 1999;57:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 110] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | American Society of Clinical Oncology. 1997 update of recommendations for the use of tumor markers in breast and colorectal cancer. J Clin Oncol. 1998;16:793-795. [PubMed] |
| 7. | Gärtner F, David L, Seruca R, Machado JC, Sobrinho-Simões M. Establishment and characterization of two cell lines derived from human diffuse gastric carcinomas xenografted in nude mice. Virchows Arch. 1996;428:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Hammer RD, Vnencak-Jones CL, Manning SS, Glick AD, Kinney MC. Microvillous lymphomas are B-cell neoplasms that frequently express CD56. Mod Pathol. 1998;11:239-246. [PubMed] |
| 9. | Huang CW, Bai L. [Clinical value of carbohydrate antigen 50 and carbohydrate antigen 242 in the diagnosis of colorectal carcinoma]. Diyi Junyi Daxue Xuebao. 2002;22:1116-1118. [PubMed] |
| 10. | Zhang S, Ma Y, Yang X. [The diagnostic values of CA242 combining other tumor markers for lung cancer]. Zhonghua Jiehe He Huxi Zazhi. 1999;22:271-273. [PubMed] |
| 11. | Dall'Olio F, Chiricolo M, Mariani E, Facchini A. Biosynthesis of the cancer-related sialyl-alpha 2,6-lactosaminyl epitope in colon cancer cell lines expressing beta-galactoside alpha 2,6-sialyltransferase under a constitutive promoter. Eur J Biochem. 2001;268:5876-5884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Elpek GO, Gelen T, Karpuzoglu G, Karpuzoglu T, Aksoy NH, Keles N. Clinicopathologic evaluation of CDw75 antigen expression in colorectal adenocarcinomas. Pathol Oncol Res. 2002;8:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Nakagoe T, Sawai T, Tsuji T, Jibiki MA, Nanashima A, Yamaguchi H, Yasutake T, Ayabe H, Arisawa K, Ishikawa H. Difference in prognostic value between sialyl Lewis (a) and sialyl Lewis (x) antigen levels in the preoperative serum of gastric cancer patients. J Clin Gastroenterol. 2002;34:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Futamura N, Nakamura S, Tatematsu M, Yamamura Y, Kannagi R, Hirose H. Clinicopathologic significance of sialyl Le (x) expression in advanced gastric carcinoma. Br J Cancer. 2000;83:1681-1687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Nakagoe T, Fukushima K, Sawai T, Tsuji T, Jibiki M, Nanashima A, Tanaka K, Yamaguchi H, Yasutake T, Ayabe H. Increased expression of sialyl Lewis (x) antigen in penetrating growth type A early gastric cancer. J Exp Clin Cancer Res. 2002;21:363-369. [PubMed] |
| 16. | Nakagoe T, Sawai T, Tsuji T, Jibiki M, Nanashima A, Yamaguchi H, Yasutake T, Ayabe H, Arisawa K, Ishikawa H. Pre-operative serum levels of sialyl Tn antigen predict liver metastasis and poor prognosis in patients with gastric cancer. Eur J Surg Oncol. 2001;27:731-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Dunphy CH, Polski JM, Lance Evans H, Gardner LJ. Paraffin immunoreactivity of CD10, CDw75, and Bcl-6 in follicle center cell lymphoma. Leuk Lymphoma. 2001;41:585-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |