Published online Jan 1, 2004. doi: 10.3748/wjg.v10.i1.77
Revised: July 20, 2003
Accepted: July 24, 2003
Published online: January 1, 2004
AIM: To observe the possible effects of transforming growth factor (TGF) β1, interleukin (IL)-6, tumor-necrosis factor (TNF) α and IL-10 on experimental rat hepatic fibrosis.
METHODS: One hundred SD rats were divided randomly into the three groups. Control group received intraperitoneal injection of saline (2 mL·kg-1), twice a week. Fibrogenesis group was injected intraperitoneally with 50% carbon tetrachloride (CCl4) (2 mL·kg-1) twice a week. Fibrosis-intervention group was given IL-10 at a dose of 4 μg·kg-1 20 minutes before CCl4 administration from the third week. At the fifth, seventh, and ninth weeks, 7 to 10 rats in each group were sacrificed to collect serum. Levels of TGF-β1, TNF-α, IL-6 and IL-10 were determined by enzyme-linked immunosorbent assay (ELISA). The liver tissues were taken for routine histological examination.
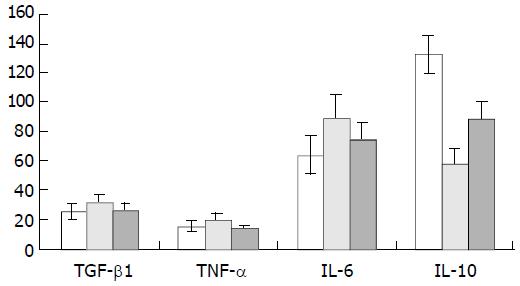
RESULTS: Hepatic fibrosis was developed with the injection of CCl4. Values of the circulating TGFβ1, TNFα, IL-6 and IL-10 in the control group were 25.49 ± 5.56 ng·L-1, 15.18 ± 3.83 ng·L-1, 63.64 ± 13.03 ng·L-1 and 132.90 ± 12.13 ng·L-1, respectively. Their levels in the CCl4-intoxication group were 31.13 ± 6.41 ng·L-1, 18.91 ± 5.31 ng·L-1, 89.08 ± 25.39 ng·L-1 and 57.63 ± 18.88 ng·L-1, respectively, and those in the IL-10-intervention group were 26.11 ± 5.32 ng·L-1,13.99 ± 1.86 ng·L-1, 74.71 ± 21.15 ng·L-1 and 88.19 ± 20.81 ng·L-1, respectively. A gradual increase was observed in the levels of TGFβ1, TNFα and IL-6 during hepatic fibrogenesis. These changes were partially reversed by simultaneous administration of IL-10. The histological parameters, characterized by CCl4-intoxification, also seemed to be improved with IL-10 treatment, the collagen production was reduced at the ninth week and the histological activity index was decreased from 7.9 ± 1.2 to 4.7 ± 0.9.
CONCLUSION: TGFβ1, TNFα and IL-6 may play important roles during CCl4-induced hepatic fibrogenesis, and IL-10 may counterbalance their effects.
- Citation: Zhang LJ, Yu JP, Li D, Huang YH, Chen ZX, Wang XZ. Effects of cytokines on carbon tetrachloride-induced hepatic fibrogenesis in rats. World J Gastroenterol 2004; 10(1): 77-81
- URL: https://www.wjgnet.com/1007-9327/full/v10/i1/77.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i1.77
Hepatic fibrosis is a common process of chronic liver injuries, characterized by increased deposition and altered composition of extracellular matrix (ECM)[1-3]. Its final stage is cirrhosis, with the liver architecture distorted by collagen bands and formation of islands of regenerating parenchymal cells[4,5]. Advanced fibrosis and cirrhosis are generally considered irreversible conditions. Many of the cellular mechanisms have been associated to hepatic fibrosis. Cytokines are soluble autocrine and paracrine mediators[6]. Expression of several cytokines has been described in human liver diseases and experimental liver injuries. Carbon tetrachloride (CCl4) is a hepatotoxin, causing liver necrosis, fibrosis and cirrhosis when administered sequentially. Hepatotoxicity is thought to involve two phases[7]. First, CCl4 is metabolized by cytochrome P450 in hepatocytes, giving rise to highly reactive trichloromethyl radicals. Second, inflammatory responses caused by CCl4 play an important role. In the latter process, some hepatic cells, including Kupffer cells (KCs), hepatic stellate cells (HSCs) and sinusoidal endothelial cells (SECs), are activated to secrete cytokines which mediate the liver fibrogenesis. Several functions have been attributed to cytokines, including activation of HSCs, modulating expression and deposition of matrix proteins and regulating the regeneration of hepatocytes. Therefore, resolution of liver fibrosis could be associated with the downregulation of inflammatory responses mediated by cytokines[8,9]. Among the cytokines, transforming growth factor (TGF) β1 is associated to the activation of HSC and the following production of ECM[10]. Tumor-necrosis factor (TNF) α and interleukin (IL)-6 are considered major hepatotoxicity mediators in several experimental models of liver injuries[11]. IL-10 can modulate the inflammatory response and alleviate hepatotoxicity[12]. In the present study, levels of circulating TGFβ1, TNF-α, IL-6 and IL-10 were measured to investigate their possible roles during CCl4-induced hepatic fibrogenesis in rats.
One hundred SD rats, weighing 140 to 180 g, were divided randomly into control (n = 24), fibrogenesis (n = 40) and fibrosis-intervention groups (n = 36). All rats were bred under routine conditions (room temperature, 22 °C ± 2 °C; humidity, 55% ± 5%; light, 12 hrs per day; drinking tap water and eating in any time; animal feed was provided by BK Company in Shanghai, China). The control rats were injected intraperitoneally with saline at a dose of 2 mL·kg-1, twice a week. The rats in the other groups received intraperitoneal injection of 50% CCl4 (2 mL·kg-1), twice a week, as described previously[13]. From the third week, the rats in intervention group were given intraperitoneally IL-10 (4 μg·kg-1, dissolved in saline) 20 minutes before CCl4 administration, as proposed by Nelson et al[14]. All injections were performed at Monday and Thursday, with their body weights determined before each injection. In the fifth week, 3 rats in the fibrogenesis group and 2 in the intervention group died. In the seventh week, 8 and 4 animals in these two groups died. In the ninth week, 10 and 6 died. At this time point, 3 rats in the control group also died. In the fifth, seventh and ninth weeks, 7 to 10 rats in each group were sacrificed to collect plasma from the common carotid artery and their liver samples.
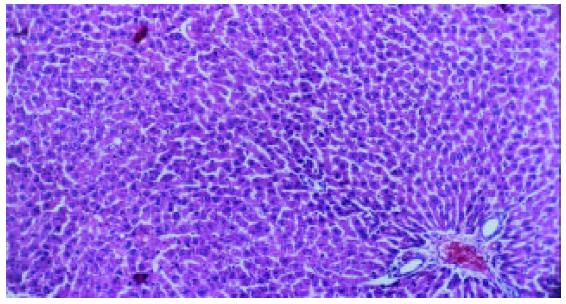
The formalin-fixed liver tissues were embedded in paraffin. Sections were stained with hemotoxylin and eosin (HE) and examined under a light microscope independently by two pathologists. Stages of fibrosis were assessed using a semi-quantitative score method as described previously. Histological activity index (HAI) was evaluated using a numerical system proposed by Knodell et al[15].
Serum was collected by centrifugation at 4 °C and frozen till use. The levels of TGF-β1, TNF-α, IL-6 and IL-10 were measured by ELISA using the kits following the manufacture’s instructions (Endogen Company, USA). Briefly, diluted serum samples were added in duplicate to 96-well plates coated with antibody and incubated at 37 °C for 2 hours. After each well was washed five times with washing buffer, peroxidase-labeled secondary antibody was added to each well and the plate was incubated at 37 °C for 1 hour. After each well was washed in a similar manner, the plate was incubated with tetramethylbenzine at room temperature for 20 minutes. The reaction was stopped by adding 1 N sulfuric acid. Optical density was measured at 450 nm using a spectrophotometric reader. Sample concentration was accessed by a standard curve.
All data were expressed as (-χ±s, t test was used for comparison between groups. P values less than 0.05 were regarded as statistically significance.
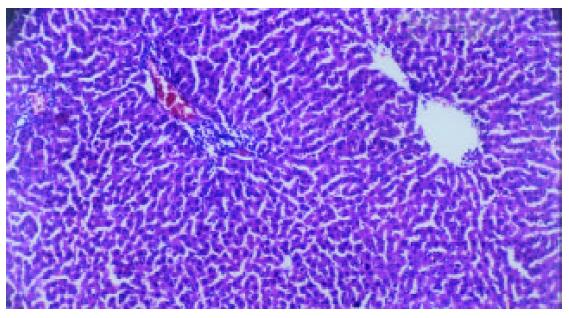
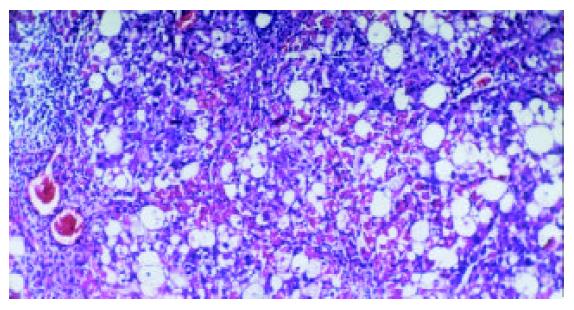
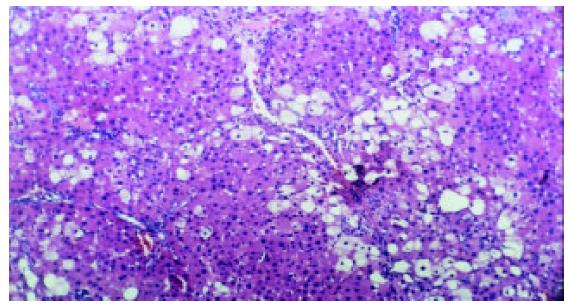
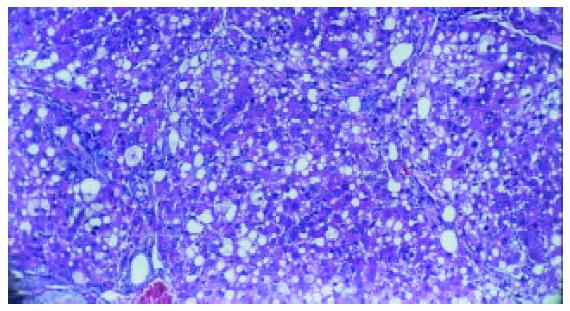
Liver fibrosis, as shown histologically, became remarkable during the treatment with CCl4. In the fifth week, steatosis and ballooning degeneration were obvious. In the seventh week, the collagen fibers increased and began to extend to the parenchyma. In the ninth week, complete fibrous septa were seen and psedolobular structures were also present occasionally. In the IL-10-intervention group, the CCl4-caused alterations as described above seemed to be markedly alleviated, with no evident changes observed in the fifth week, less profound steatosis and necrosis noted in the seventh week, and only early-stage fibrosis found in the ninth week. HAI decreased from 7.9 ± 1.2 in the fibrogenesis group to 4.7 ± 0.9 in the IL-10-intervention group (P < 0.05) (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5).
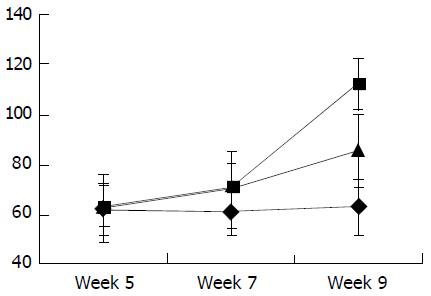
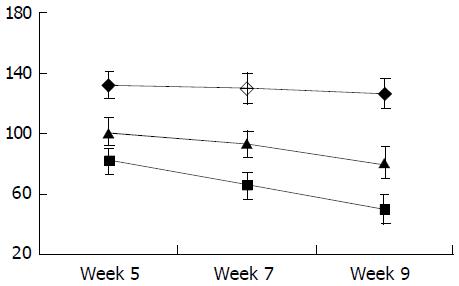
As shown in Figure 6, the level of circulating IL-10 was lower in fibrogenesis group than in the control group (P < 0.05). The levels of TGF-β1, TNF-α and IL-6 in were higher in fibrogenesis group than in the control group (P < 0.05). However, values of these three cytokines were significantly reduced after the intervention treatment with IL-10 (P < 0.05), being similar to the levels in the control group (P > 0.05). Therefor, serum concentrations of TGF-β1, IL-6 and TNF-α were increased during CCl4-caused hepatic fibrogenesis.(Table 1)
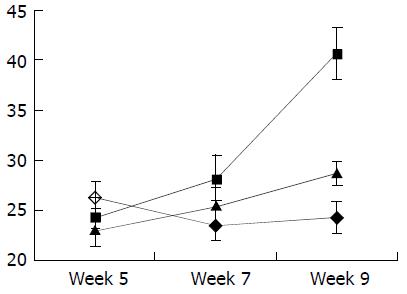
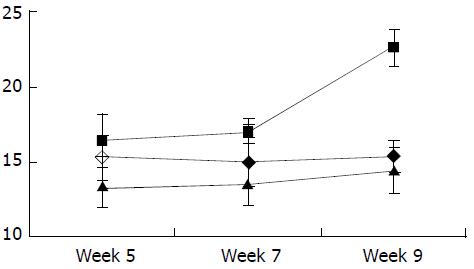
As shown in Figure 7, Figure 8, Figure 9, Figure 10, concentrations of TGF-β1, TNF-α and IL-6 were gradually increased along with CCl4-intoxification (P < 0.05). These changes were partially reversed by the treatment with IL-10, particularly in the ninth week (P < 0.05).
Previous data have shown that rats chronically exposed to CCl4 were resistant to cirrhosis. In rats, exposed to CCl4 for 8 to 12 weeks could result in localized fibrosis[16]. In the present study, fully developed hepatic fibrosis was observed in the rats after 9 weeks of CCl4 intoxication. The contents of cytokines in serum were found to vary during the process. Cytokines constitute a complex network involved in the regulation of inflammatory responses and homeostasis of organ functions. Following liver injury, a wound healing process evolves, including proliferation of surrounding hepatocytes, proliferation and differentiation of stem cells, and conversion of parasinusoidal cells into activated HSCs capable of driving the accumulation of ECM. If the injuries were persistent or the wound healing process was aberrant, the final phenotype might be a fibrotic, dysfunctional liver[8]. A number of cytokines and growth factors could augment or inhibit the fibrotic response to injuries[18]. Accumulating data indicate that HSCs are the major source of fibrillar and non-fibrillar matrix proteins during hepatic fibrogenesis. When activated, HSCs could proliferate and transform to a myofibroblast-like phenotype, expressing α-smooth muscle actin (α-SMA) and collagen types I, III and IV, fibronectin and proteoglycan[19]. It has been found several cytokines play roles by acting on HSC, and among them, TGF-β1 is one of the most important cytokines involved in the fibrotic and cirrhotic transformation of the liver[20-23]. After a fibrogenic injury, expression of three forms of TGF-β is greatly upregulated in HSCs. It was reported that administration of TGF-β1in vivo induced an inflammatory reaction, and knock-out of TGF-β1 gene resulted in widespread inflammatory diseases. Therefore, TGF-β1 may have a pro-inflammatory effect.
TNF-α was originally identified as a circulating factor that resulted in remarkable hemorrhage and necrosis of tumors when administered to tumor-bearing mice. It has been implicated in a number of liver diseases and is an important mediator of many physiological conditions. Several evidences suggest that TNF-α is among the most crucial components in the early signaling pathways leading to regeneration. However, there has been evidence that TNF-α is a primary endogenous factor mediating acute inflammatory conditions such as endotoxic shock. It appears that inhibition of the TNF-α effect might have a broader clinical application value than expected previously[26]. In several animal models of immune-mediated liver injury or hepatotoxin sensitization, TNF-α administration could lead to hepatocyte apoptosis and liver failure[27]. Besides its modulation effect on ECM production, TNF-α has been considered a mediator of cell injuries in liver caused by alcoholism, reperfusion, primary graft nonfunctional, graft rejection and endotoxic insult[28,29]. TNF-α is expressed by both infiltrating inflammatory cells and hepatocytes in chronic liver injuries, and it has been proposed to play an important role during tissue damage[30]. Our data provide a further evidence for the role during hepatic fibrosis.
The role of IL-6 during chronic liver injuries and fibrogenesis remains to be clarified. Some reports provided evidences for an important role of IL-6 in reducing CCl4-induced acute and chronic liver injury and fibrosis[31-33]. However, some other data showed that the serum level of IL-6 was associated with hepatic necroinflammatory activity in patients with chronic hepatitis and cirrhosis[34,35]. Our results support the latter view. Animal experiments showed that IL-6 was associated to activated HSCs during acute and chronic injuries, indicating that IL-6 is a responsive element to liver injuries. Moreover, CCl4-induced expression of TGF-β1 and hepatocyte growth factor in liver was shown to be associated with the serum level of IL-6. Thus, IL-6 might be vitally involved in fibrotic changes, partly by modulating intrahepatic expression of other cytokines[36,37].
Recent studies have suggested a protective role of IL-10 during liver transplantation and experimental liver injuries induced by galactosamine and lipopolysaccharide[38]. However, the mechanism of the protective effects is not fully understood. IL-10 is a potent anti-inflammatory cytokine that inhibits the synthesis of pro-inflammatory cytokines by T helper type 1 cells. It is produced locally in the liver and acts in an autocrine or a paracrine manner. Previous reports indicated that IL-10 had some role in remodeling ECM[39,40]. IL-10 was shown to downregulate the synthesis of collagen type I and to upregulate expression of metalloproteinase. It could also play an antifibrogenic role by downregulating the profibrogenic cytokines including TGF-β1 and TNF-α[11,41]. Nelson et al treated 24 patients with chronic hepatitis C using IL-10, and found that IL-10 administration resulted in reduction of the serum ALT level, partial resolution of the hepatic inflammation and alleviation of the fibrosis[14]. IL-10 may be useful in the treatment of chronic liver diseases regarding the prevention of advanced fibrosis and cirrhosis[42].
Edited by Su Q and Wang XL
| 1. | Friedman SL. Molecular mechanisms of hepatic fibrosis and principles of therapy. J Gastroenterol. 1997;32:424-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 91] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Du WD, Zhang YE, Zhai WR, Zhou XM. Dynamic changes of type I,III and IV collagen synthesis and distribution of collagen-producing cells in carbon tetrachloride-induced rat liver fibrosis. World J Gastroenterol. 1999;5:397-403. [PubMed] |
| 3. | Wang JY, Guo JS, Yang CQ. Expression of exogenous rat collagenase in vitro and in a rat model of liver fibrosis. World J Gastroenterol. 2002;8:901-907. [PubMed] |
| 4. | Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 886] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 5. | Abdel-Aziz G, Lebeau G, Rescan PY, Clément B, Rissel M, Deugnier Y, Campion JP, Guillouzo A. Reversibility of hepatic fibrosis in experimentally induced cholestasis in rat. Am J Pathol. 1990;137:1333-1342. [PubMed] |
| 6. | Nakamura T, Sakata R, Ueno T, Sata M, Ueno H. Inhibition of transforming growth factor beta prevents progression of liver fibrosis and enhances hepatocyte regeneration in dimethylnitrosamine-treated rats. Hepatology. 2000;32:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 207] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Shi J, Aisaki K, Ikawa Y, Wake K. Evidence of hepatocyte apoptosis in rat liver after the administration of carbon tetrachloride. Am J Pathol. 1998;153:515-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 190] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Friedman SL. Cytokines and fibrogenesis. Semin Liver Dis. 1999;19:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 262] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 9. | Koziel MJ. Cytokines in viral hepatitis. Semin Liver Dis. 1999;19:157-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 164] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Bissell DM, Roulot D, George J. Transforming growth factor beta and the liver. Hepatology. 2001;34:859-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 284] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 11. | Kovalovich K, DeAngelis RA, Li W, Furth EE, Ciliberto G, Taub R. Increased toxin-induced liver injury and fibrosis in interleukin-6-deficient mice. Hepatology. 2000;31:149-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 243] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 12. | Yoshidome H, Kato A, Edwards MJ, Lentsch AB. Interleukin-10 suppresses hepatic ischemia/reperfusion injury in mice: implications of a central role for nuclear factor kappaB. Hepatology. 1999;30:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 180] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Morrow JD, Awad JA, Kato T, Takahashi K, Badr KF, Roberts LJ, Burk RF. Formation of novel non-cyclooxygenase-derived prostanoids (F2-isoprostanes) in carbon tetrachloride hepatotoxicity. An animal model of lipid peroxidation. J Clin Invest. 1992;90:2502-2507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 251] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Nelson DR, Lauwers GY, Lau JY, Davis GL. Interleukin 10 treatment reduces fibrosis in patients with chronic hepatitis C: a pilot trial of interferon nonresponders. Gastroenterology. 2000;118:655-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 245] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 15. | Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology ;. 1981;1:431-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2558] [Cited by in RCA: 2508] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 16. | Tsukamoto H, Matsuoka M, French SW. Experimental models of hepatic fibrosis: a review. Semin Liver Dis. 1990;10:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 193] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Missale G, Ferrari C, Fiaccadori F. [Cytokine mediators in acute inflammation and chronic course of viral hepatitis]. Ann Ital Med Int. 1995;10:14-18. [PubMed] |
| 18. | Davis BH, Kresina TF. Hepatic fibrogenesis. Clin Lab Med. 1996;16:361-375. [PubMed] |
| 19. | Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 828] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 20. | Nakamura T, Sakata R, Ueno T, Sata M, Ueno H. Inhibition of transforming growth factor beta prevents progression of liver fibrosis and enhances hepatocyte regeneration in dimethylnitrosamine-treated rats. Hepatology. 2000;32:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 207] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 21. | Dooley S, Delvoux B, Lahme B, Mangasser-Stephan K, Gressner AM. Modulation of transforming growth factor beta response and signaling during transdifferentiation of rat hepatic stellate cells to myofibroblasts. Hepatology. 2000;31:1094-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 191] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | García-Trevijano ER, Iraburu MJ, Fontana L, Domínguez-Rosales JA, Auster A, Covarrubias-Pinedo A, Rojkind M. Transforming growth factor beta1 induces the expression of alpha1(I) procollagen mRNA by a hydrogen peroxide-C/EBPbeta-dependent mechanism in rat hepatic stellate cells. Hepatology. 1999;29:960-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 190] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 23. | Si XH, Yang LJ. Extraction and purification of TGFbeta and its effect on the induction of apoptosis of hepatocytes. World J Gastroenterol. 2001;7:527-531. [PubMed] |
| 24. | Bissell DM, Wang SS, Jarnagin WR, Roll FJ. Cell-specific expression of transforming growth factor-beta in rat liver. Evidence for autocrine regulation of hepatocyte proliferation. J Clin Invest. 1995;96:447-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 321] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 25. | Knittel T, Janneck T, Müller L, Fellmer P, Ramadori G. Transforming growth factor beta 1-regulated gene expression of Ito cells. Hepatology. 1996;24:352-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 76] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Llorent L, Richaud-Patin Y, Alcocer-Castillejos N, Ruiz-Soto R, Mercado MA, Orozco H, Gamboa-Domínguez A, Alcocer-Varela J. Cytokine gene expression in cirrhotic and non-cirrhotic human liver. J Hepatol. 1996;24:555-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Zhang GL, Wang YH, Teng HL, Lin ZB. Effects of aminoguanidine on nitric oxide production induced by inflammatory cytokines and endotoxin in cultured rat hepatocytes. World J Gastroenterol. 2001;7:331-334. [PubMed] |
| 28. | Roberts RA, James NH, Cosulich SC. The role of protein kinase B and mitogen-activated protein kinase in epidermal growth factor and tumor necrosis factor alpha-mediated rat hepatocyte survival and apoptosis. Hepatology. 2000;31:420-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Crespo J, Cayón A, Fernández-Gil P, Hernández-Guerra M, Mayorga M, Domínguez-Díez A, Fernández-Escalante JC, Pons-Romero F. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001;34:1158-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 503] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 30. | Hernandez-munoz I, de La Torre P, Sanchez-Alcazar JA, Garcia I, Santiago E, Munoz-Yague MT, Solis-Herruzo JA. Tumor ne-crosis factor inhibits collagen 1 (1) gene expression in rat he-patic stellate cells through a G protein. Gastroenterology. 1997;113:625-640. [RCA] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Selzner M, Camargo CA, Clavien PA. Ischemia impairs liver regeneration after major tissue loss in rodents: protective effects of interleukin-6. Hepatology. 1999;30:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 121] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Sakamoto T, Liu Z, Murase N, Ezure T, Yokomuro S, Poli V, Demetris AJ. Mitosis and apoptosis in the liver of interleukin-6-deficient mice after partial hepatectomy. Hepatology. 1999;29:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 205] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 33. | Peters M, Blinn G, Jostock T, Schirmacher P, Meyer zum Buschenfelde KH, Galle PR, Rose-John S. Combined interleukin-6 and soluble interleukin-6 receptor accelerates murine liver regeneration. Gastroenterol. 2000;119:1663-1671. [RCA] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Wang JY, Wang XL, Liu P. Detection of serum TNF-alpha,IFN-beta,IL-6 and IL-8 in patients with hepatitis B. World J Gastroenterol. 1999;5:38-40. [PubMed] |
| 35. | Zhen Z, Zhou JY, Liu JX, Hong ZJ, Pei X. Relationship between IL-6 and SIL-6R and their pathogecicity in viral hepatitis. Shijie Huaren Xiaohua Zazhi. 2000;8:1434-1435. |
| 36. | Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1213] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 37. | Natsume M, Tsuji H, Harada A, Akiyama M, Yano T, Ishikura H, Nakanishi I, Matsushima K, Kaneko S, Mukaida N. Attenuated liver fibrosis and depressed serum albumin levels in carbon tetrachloride-treated IL-6-deficient mice. J Leukoc Biol. 1999;66:601-608. [PubMed] |
| 38. | Louis H, Le Moine O, Peny MO, Gulbis B, Nisol F, Goldman M, Devière J. Hepatoprotective role of interleukin 10 in galactosamine/lipopolysaccharide mouse liver injury. Gastroenterology. 1997;112:935-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Reitamo S, Remitz A, Tamai K, Uitto J. Interleukin-10 modulates type I collagen and matrix metalloprotease gene expression in cultured human skin fibroblasts. J Clin Invest. 1994;94:2489-2492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 145] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | Thompson K, Maltby J, Fallowfield J, McAulay M, Millward-Sadler H, Sheron N. Interleukin-10 expression and function in experimental murine liver inflammation and fibrosis. Hepatology. 1998;28:1597-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 189] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 41. | Louis H, Van Laethem JL, Wu W, Quertinmont E, Degraef C, Van den Berg K, Demols A, Goldman M, Le Moine O, Geerts A. Interleukin-10 controls neutrophilic infiltration, hepatocyte proliferation, and liver fibrosis induced by carbon tetrachloride in mice. Hepatology. 1998;28:1607-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 209] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 42. | Wang XZ, Zhang LJ, Li D, Huang YH, Chen ZX, Li B. Effects of transmitters and interleukin-10 on rat hepatic fibrosis induced by CCl4. World J Gastroenterol. 2003;9:539-543. [PubMed] |